Onvansertib will not add much value as first line therapy for patients with RAS-mutant metastatic colorectal cancer
Why Cardiff Oncology's July 29, 2025 readout will not be a win
The July 29 readout from Cardiff Oncology will be on the following trial: Onvansertib (third-generation oral PLK1i, 20 or 30mg [2 different groups] once daily on days 1-5 and 15-19 of 28-day cycle) + bevacizumab (VEGFi) + FOLFOX/FOLFIRI given on days 1 and 15 of each 28 day treatment cycle as first line (1L) treatment of KRAS or NRAS mutant metastatic colorectal cancer. It will be compared to FOLFOX/FOLFIRI + bevacizumab which is current standard of care. Will the addition of onvansertib make a significant difference in overall response rate and median progression free survival?
Cardiff has ~113 patients across 6 arms. This is ~19 patients per arm. But, we can collapse the chemotherapy groups (FOLFOX and FOLFIRI) into one. They are relatively interchangeable. This means 38 patients per group (chemo + VEGFi + onvansertib 20 mg, chemo + VEGFi + onvansertib + 30 mg, chemo + VEGFi).
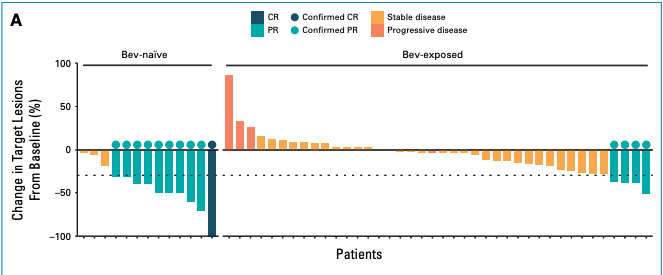
They released some interim data from the current study which we will cover later. First, let us consider their 2024 JCO paper describing onvansertib + chemo + VEGFi as 2L treatment. They treated 53 KRAS mutant metastatic CRC (mCRC) patients with onvansertib (PLK1i 15 mg/m2 on days 1-5 and 15-19) + FOLFIRI + VEGFi. These 53 patients had previously received at least 6 weeks of oxaliplatin and fluoropyrimidine, with or without bevacizumab, and either progressed within 6 months of treatment or shown intolerance to oxaliplatin. Functionally, these patients were resistant to their initial therapy. The overall response rate (ORR) was 26.4% for the 53 patients. Not great. Then, Cardiff looked post-hoc at the subset of patients who were VEGFi-naive. In the study, 13 patients received only prior chemo and 40 patients received VEGFi and chemo. Why were these 13 RAS-mutant mCRC patients not given VEGFi? Perhaps the RAS mutation was not detected at that time, uncontrolled hypertension, recent major surgery, or their disease was not metastatic. Something changed to rule them back into VEGFi because clearly these patients were eligible for VEGFi when Cardiff was recruiting. Within prior chemo-only patients, 10 of 13 had > 30% reduction in target lesion size compared to baseline (an objective response by RECIST) when treated with VEGFi+PLKi+chemo. Only 4 of 40 prior VEGFi exposed patients responded to the new treatment. These patients were already resistant to VEGFi so additional VEGFi was not going to do anything. The addition of PLK1i did not add much value here. Whatever mechanism makes a patient in Cardiff’s target population resistant to VEGFi also renders PLK1i ineffective. The hypothesis is that VEGFi and PLK1i given at the same time acts synergistically to produce deep and significant responses. This idea is similar to other dual blockade ideas like BRAFi and MEKi.
Cardiff then surmised that since 10 of 13 responded when given VEGFi for the first time and PLK1i, their treatment should be used in combination with VEGFi as a first line treatment. But, there was a group of patients who never qualified for the trial - those that responded to VEGFi and chemo. What is their ORR? This is equivalent to us asking what the ORR for first line VEGFi and chemo is for RAS-mutant mCRC patients? This informs the standard of comparison onvansertib will have to beat.
What is the response of chemo + VEGFi in RAS mt mCRC 1L? The BECOME trial, which enrolled only patients with RAS mutant, unresectable, liver-limited metastatic colorectal cancer, reported an objective response rate of 54.5% for bevacizumab plus mFOLFOX6, compared to 36.7% for mFOLFOX6 alone. Similarly, a randomized trial by Avallone et al. found an objective response rate of ~53% in RAS mutant subgroups treated with oxaliplatin-based chemotherapy plus bevacizumab. The FIRE-3 trial when subset to RAS-mutant mCRC had an ORR of 48%. So, we can expect a low-50s response rate for the control group in the upcoming readout. Will onvansertib beat is such that its confidence interval largely does not overlap this standard of care?
I do not think prior chemo in RAS mutant mCRC takes away the opportunity for a patient to have a objective response to VEGFi + PLK1i + chemo. So, the experimental arm in the upcoming readout should function much like the prior chemo only treatment group in their JCO reported results. ORR was 77% (CI 46% - 95%) and PFS was 15 months. If 8 of the 13 (so two less than observed) had an objective response then the ORR is about equal to that of 1L chemo + VEGFi. The issue with objective responses is that a patient can have a 30% target lesion decrease but recur shortly thereafter and it still counts. Progression-free survival or better yet, overall survival is a better basis for comparison. More importantly, in the KM curve of PFS, 6 patients in the bev-naive group were censored < 12 months from start date. 8 were censored in total. The effective sample size is 5 which makes it near impossible to tell what is going on with the actual PFS. In comparison only 8 of the 40 were censored in the prior VEGFi group. This is a more stable estimate.
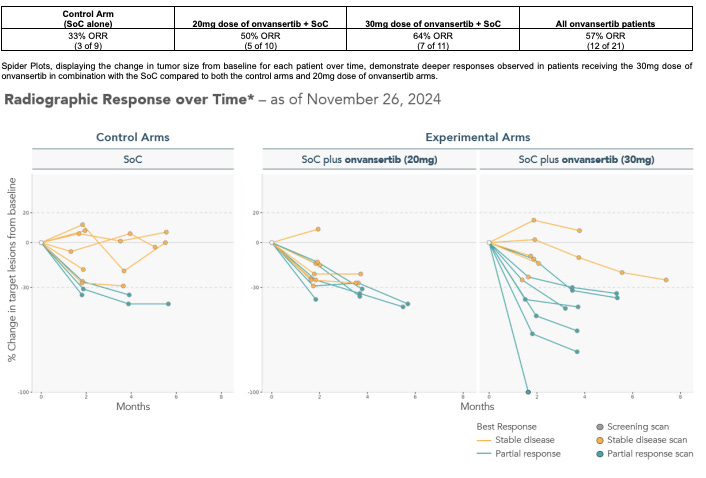
Now, let us consider their Nov 26, 2024 update of the trial we will be getting results from on July 29. Results shown below.
3 of 9 responded in the standard of care. Consider that there is a patient with 29% change in the standard of care arm which will hit 30% in the next 6 months - the real ORR is 4 of 9 in this readout. This is well below our expected mid-50s so expect that to change when more patients are enrolled. Now, consider the patient in the 30 mg group with 100% response. This smells like the patient in the 2024 JCO study who enrolled in the expanded access program to continue receiving treatment and likely is an outlier relative to the other data observed in the paper. So perhaps the true ORR in the 30 mg group in the interim data presented is 6 of 11 (ORR 54%). This is in line with expected standard of comparison. And consider that the patient with ~5 months of follow up at just barely over 30% decrease could have easily flipped either way making the ORR 45%.
Let us look at this a different way. Assume onvansertib 30 mg treated group ORR is 64% like reported in the data above. Assume standard of care is on the lower end of what is shown in previous RCTs at 52%. We have 38 patients in the 30 mg group and 38 patients in the standard of care. We can use an inverse beta with alpha of 0.05 to compute confidence intervals for the ORR. So, the ORR CI for 30 mg is 48-80% and control is 33-67%. The confidence intervals have lots of overlap.
Dosage in 2024 JCO study was 15 mg/m2. At 20 mg, the assumed body surface area (BSA) is 1.3 and at 30 mg, the assumed body surface area is 2. Average BSA for men is 1.9-2 and women is 1.6-1.7. So, the JCO dose is closer to 30 mg than 20 mg. We can expect the 30 mg to perform like the 15 mg/m2 would. The pharmacokinetics have also been called into question. The pulse dosing and lower dose are probably there to avoid myelosuppression safety concerns noted with other PLK1i drugs (which ended up killing them). So, Cardiff is sacrificing efficacy here to avoid bad myelosuppression.
Cardiff postponed the release of the data we are about to see. Why did they prolong releasing their data and switch their CMO in between? Were they looking for more data to come in to see if they can show some signal? Some people have claimed that the new CMO is a good thing. I interpret it as having no bearing on the underlying trial. Sure, he has experience in a positive phase 3 trial for mCRC but the previous CMO was no slouch. I could even argue that the new CMO is a negative signal. Long story short, two companies had a new CMO appointed 2-3 months before a phase 2 or 3 readout (TARA and UBX). TARA fell 19% after readout and UBX fell 33% after readout. Opthea (OPT) hired a new CEO 6 months before readout and their Ph3 trial failed and the company went to zero.
Conclusion
Onvansertib will be at best a very thin additional benefit to 1L chemo + VEGFi. With 38 patients, the confidence interval for ORR will surely overlap considerably with standard of care. The JCO paper that led to the new VEGFi + PLK1i synergy hypothesis in 1L RAS mutant mCRC is based on minimal clinical evidence (e.g., 5 patients constructed the PFS curve and one patient had a grade 4 colon perf) and a few wet lab/bioinformatic experiments. There are questions on the pharmacokinetics too. Net-net in the long term I do not see onvansertib in the oral pulse-dose 1L setting for RAS mutant mCRC making meaningful improvements to patient outcomes. As always, open to comments, suggestions, and where I might have reasoned incorrectly. I am happy to be wrong. If onvansertib provides benefit to patients and improves median PFS and median OS, that will be great for patients and I am all for it. Better treatment options are always a good thing.


What did you think about the updated the company released a little over a week ago?