Can a KRAS vaccine change the game in pancreatic cancer?
Cool science, soft signal
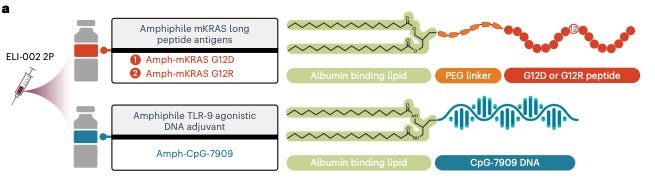
Elicio Therapeutics ($ELTX) is developing a lymph-node targeted amphiphile mutant KRAS vaccine for pancreatic cancer. They take mutant KRAS peptide fragments and link it to a lipid that binds albumin. Now, the fragment gets carried by albumin to the lymph node. They also inject CpG-DNA bound to an albumin-binding lipid to trigger the innate immune system and increase immunogenicity of the peptide fragment. These two components are given together and comprise the treatment.
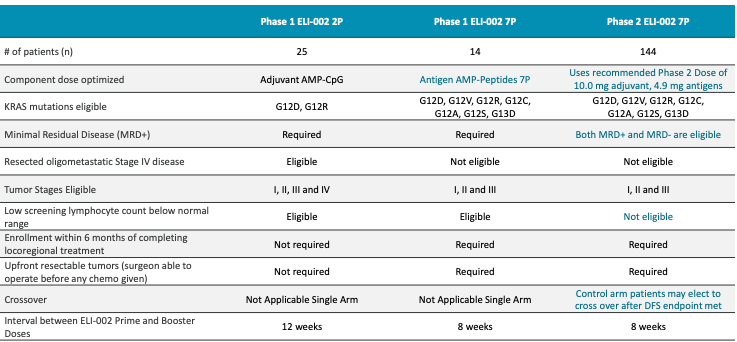
They ran AMPLIFY-201 and AMPLIFY-7P Phase 1 trials, which enrolled patients with G12D or G12R KRAS mutations who had minimal residual disease (MRD) positive pancreatic ductal adenocarcinoma (PDAC) or colorectal cancer (CRC) after locoregional therapy (e.g., surgical resection of tumor).
Let’s first look at AMPLIFY-7P results published November 2024. They enrolled KRAS G12D/R/V/C/S/A or G13D MRD+ PDAC patients and gave them CpG-DNA 10 mg with either 1.4 mg or 4.9 mg of Amph-Peptide 7P (7 mKRAS peptides linked to albumin binding moiety). 14 patients (13 PDAC, 1 CRC). No dose-limiting toxicities, or discontinuation due to AE. No cytokine release syndrome noted. They reported results for 12 patients, implying 2 patients’ data were thrown out. Maybe they left the study prior to treatment initiation or some other reason.
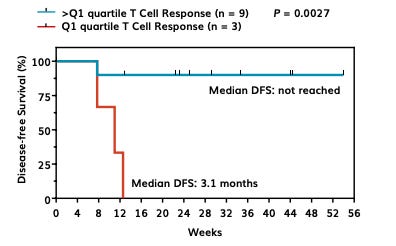
They ranked their 12 patients by T cell response and when subset to 3 worst responders versus the other 9, the worst 3 had a median disease-free survival (mDFS) of 3.1 months. This is an arbitrary splitting of the data. We should consider all 12 together. At 12 weeks, in the pooled data, there are 4 events meaning the DFS is 66%. (I know the third death in the Q1 group happens a little after 12 weeks, but we’ll just call it 12 weeks for ease of data analysis.) There are 8 patients at-risk after 12 weeks, all of whom get censored because the study is immature. There are nine ticks in the >Q1 group, suggesting nine patients were censored which doesn’t make sense since there are only 9 patients in the group and 1 had an event prior to any censoring. Anyhow, let’s be charitable and assume that was an error by an intern that didn’t get caught.
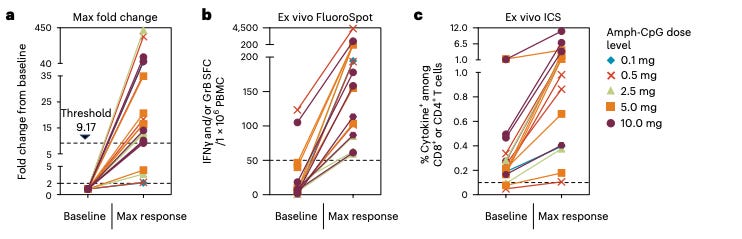
Going back to their data, higher dose of vaccine produces greater T cell response. But, counting dots in the below figure, I see 4 maroon triangles and 6 blue dots representing 10 patients. But, they report having peripheral blood samples for 12 evaluable dosed patients in their poster from which this figure was also taken. So, they threw out 2 more patients’ data in this T-cell analysis with no notice or explanation in their poster? Presumably these data points belong to 2 low dose patients to get to an even 6 and 6 patients in each dose group. The intern has made a second mistake. Evaluating the data as is, in spite of our concerns about the integrity of the data, we see that first quartile responders are made of 2 low dose and 1 high dose patients. So, that leaves 2 low dose and 5 higher dose patients in the not first quartile group. We have signal here that the 4.9mg dose has a better clinical response than 1.4 mg since non-first quartile patients only had one observed event that occurred at 8 weeks but first quartile patients all died within 12 weeks.
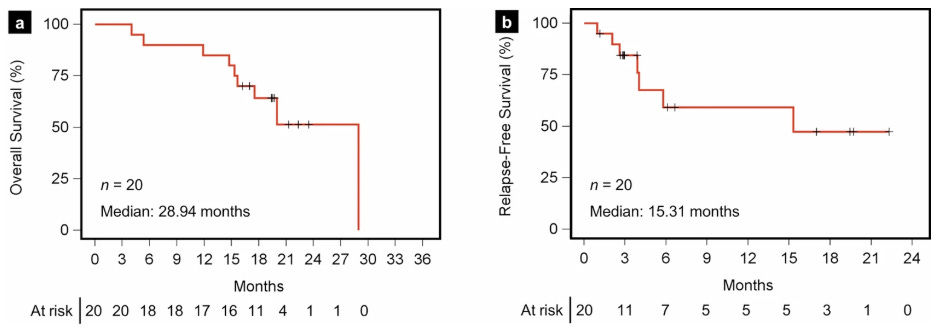
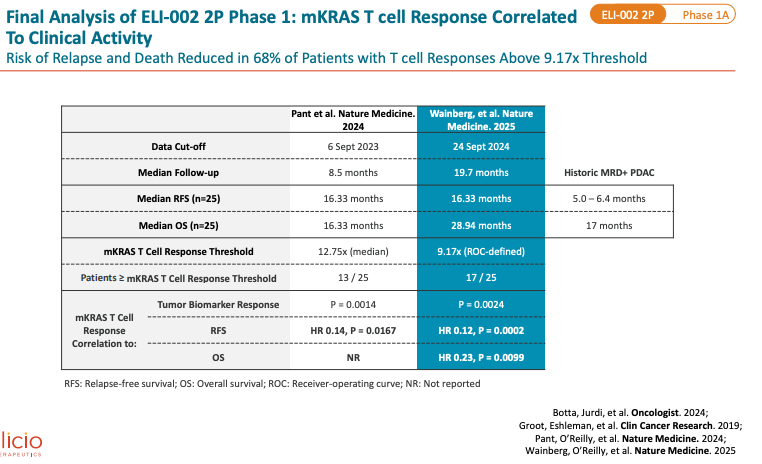
Now, let us look at AMPLIFY-201 published in Nature Medicine (first published in January 2024 and final results also presented in Nature Medicine in August 2025). Population enrolled is adjuvant PDAC (n = 20) patients (and a few CRC patients n = 5) who were MRD+ (ctDNA in 13/25 and CEA/CA199 in 5/25) with KRAS G12D or G12R mutation. This is basically the same as the 7P trial population except they no longer allowed patients with KRAS G12V/C/S/A or G13D mutants. Dose range was much more variable than 7P study, with 0.1mg, 0.5mg, 2.5mg, 5.0mg, and 10mg peptide dose levels. And, the peptide used was 2P - less potent than 7P per KOL call 2024 with Eileen M. O’Reilly (oncologist MSK). I’m assuming this means 2 mKRAS peptide fragments were given. Median of 19.7 months of follow up (2024 update had median follow up of 8.5 months), no new safety signals. Median OS was 28.94 months. Median RFS (recurrence-free survival) by imaging was 16.33 months, maintained from prior update in 2024. PDAC subset had mRFS of 15.31 months and mOS of 28.94 months (shown below).
You can’t look at the OS curve here and think the median value was truly 28.94 months. The curve skims the median hard. The real mOS probably lies between 16-20 months. We can debate where that may be, but it certainly is much less than 28 months. Median follow up was 19 months so the patients in the OS curve around 15-21 that get censored are probably censored because no more follow up available at the time of data cutoff. If any of the 7 censored prior to month 24 have an event, the mOS drops considerably. The Relapse-Free Survival (RFS) curve also skims the median here. The real mRFS is maybe somewhere from 6 to 12 months, not 15 months.
6 patients received subsequent chemotherapy following tumor biomarker increase yet remained free from progression through follow-up. 3 of six patients who remained free from radiographic progression received subsequent therapy, including ICI, following biomarker progression. So, subsequent therapy whether ICI or chemotherapy could be the key here. The argument may be that KRAS-vaccine improves response to ICI or chemotherapy but that is hard to prove with the current trial design.
They define a threshold for T cell response and there are 8 patients below the threshold and 17 patients above the threshold. A quick word on the threshold. In their first paper in 2024, they defined threshold as the median T cell response. Then, in 2025, presumably feeling that was not a robust marker, they did an ROC analysis of T cell response and radiographic progression or death (I think this was the endpoint for ROC, it hard to say what it is based off of) and for each T cell response value they computed the Youden index (sensitivity + specificity - 1) with larger values being better. This is an exploratory analysis.
All 8 below threshold progressed or died, 6 of whom got subsequent therapy. Of the 17 above threshold, 11 got subsequent therapy. Of the 11 who got subsequent therapy, 6 did not progress or die and 5 did not. Of the 6 who did not get subsequent therapy, 5 did not progress. First, this suggests that people with a really large response are going to not progress or die. We can look at individual T cell response and survival time data to confirm this. Second, subsequent therapy is offered if ctDNA levels increase which means the therapy is not working. Most people whether they were above or below threshold got subsequent therapy (17 of 25). Getting subsequent therapy is a 50-50 chance of non-progression/death if you had a good T cell response (above threshold) and a 0% chance if poor T cell response. We can read that in two ways: a) vaccines that create a strong T cell response enable the patient respond to subsequent therapy better, or b) those who respond well to vaccines are able to respond better to subsequent therapy because they have more intact immune systems at baseline, tumor microenvironment more friendly to incoming therapy (e.g., desmoplastic response is not so harsh). Option b is my most likely but I would certainly like to believe option a, I’m just not sure how to de-risk that hypothesis with the current trial design.
All 12 patients who induced both CD4+ and CD8+ T cells had T cell responses above the 9.17 threshold. This means these 12 patients strongly activated their immune systems. Perhaps they were even naturally primed and already had mKRAS recognition abilities.
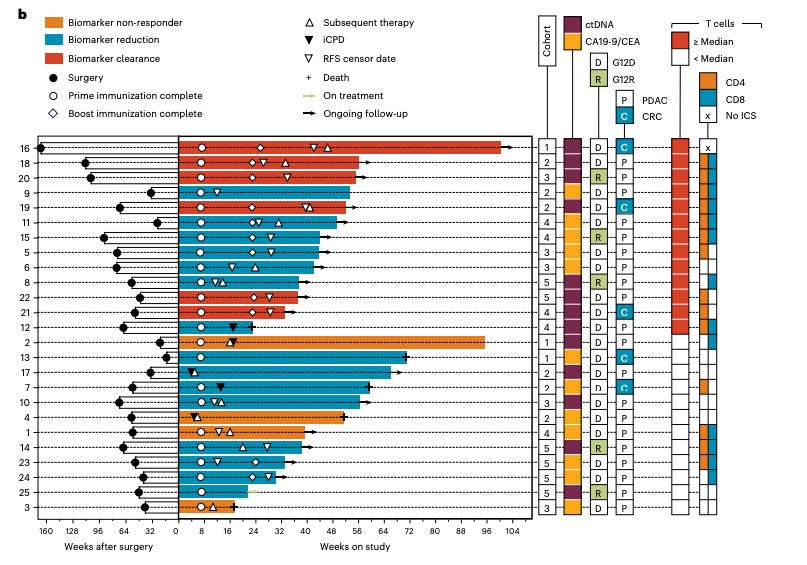
Let us study the below figure from the Nature Medicine 2024 paper, it’s filled with a bunch of great information on the trial.
First off, notice there are 25 patients here, the same as in the final analysis, we just have longer follow up in the final. Best overall biomarker response could have gotten more negative for some of these patients over time. But, if we look at the source data for Figure 1d in the 2025 paper with updated follow up and best biomarker response, we see that 6 patients had 100% clearance consistent with the 2024 waterfall plot above. So, 3 of the 6 clearance events were CRC. In the 3 PDAC clearance patients, their RFS censor date was around 24 weeks on study. And these patients shortly thereafter received subsequent therapy, per the chart. They are alive still but certainly had disease progression on therapy. And, we see that all the patients with increases in tumor markers (non-responders) were PDAC patients. They were censored for RFS quite early, around 6-10 weeks on study and received subsequent therapy. 2 of the 3 non-responders died at 16 and 48 weeks. Taken all together, the point is 1) basically everyone had recurrence and were given subsequent therapy, 2) non-responders are PDAC patients who have very poor outcomes and CRC patients enrich for better biomarker responders.
Notice that 3 of 5 biomarker clearers were CRC but only 5 of the 25 total cases were CRC. So, PDAC patients don’t respond as well as the total population. Look at the figure below, 5 patients died. 2 were CRC patients (both responders) that survived for 56+ weeks but the 3 PDAC who died, died quickly at 16 (nonresponder), ~24 (reduction), and 48 weeks (nonresponder).
I can’t really see a strong dose-response relationship in the below figure. Some patients respond really well to vaccines regardless of dose.
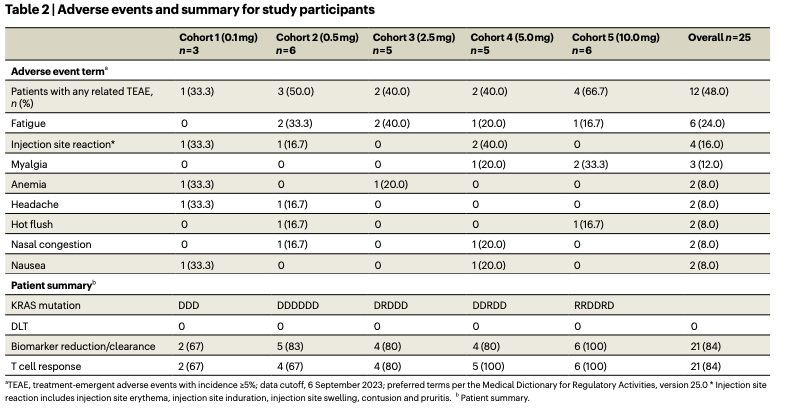
Let’s look at safety here from the 2024 paper (n = 25)
There is a dose-response relationship between adverse effect rate and dose. This dose seem like a pretty safe drug. Some anemia. Fatigue, myalgia, nasal congestion expected of something that interacts with the immune system as we so often see with basically any other vaccine.
Now let us look at company statements and what we might expect in standard of care treated patients. From Elicio Therapeutics press release:
IDMC’s positive recommendation to continue the AMPLIFY-7P study to final analysis without modification. Final DFS data [for phase 2 study] expected in Q4 this year, we are focused on advancing clinical preparation for a potential pivotal Phase 2 trial. Upon final DFS analysis, they will request and conduct End-of-Phase-2 meeting with the FDA to finalize regulatory strategy for the pivotal Phase 3 trial for ELI-002.
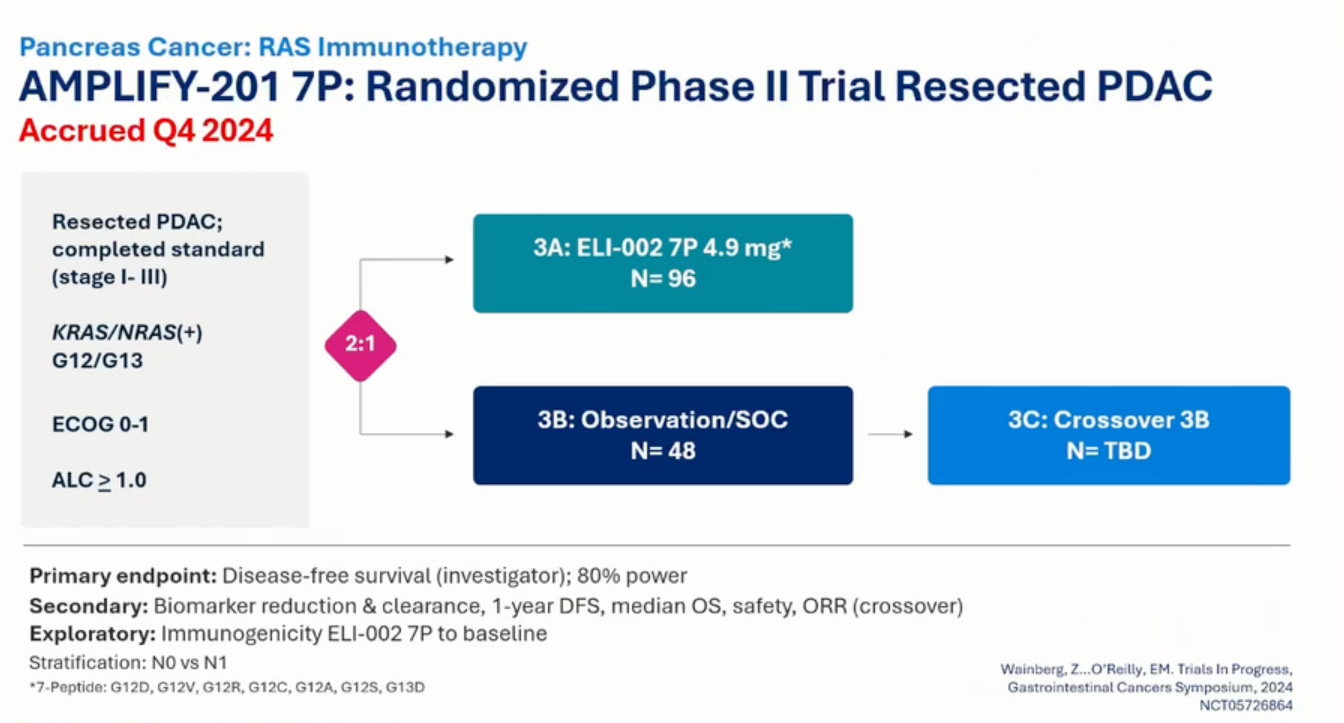
To refresh ourselves, AMPLIFY-7P is trial of (Amph-Peptide 7P) mKRAS-peptides and CpG-DNA conjugated to lipids as adjuvant treatment in PDAC patients with mKRAS.
Phase 2 of the study is PDAC patients randomized 2:1 to treatment or observation/SOC. Subjects randomized to observation will be able to elect to cross-over to treatment in the event of confirmed disease progression. Notably, patients can be MRD+ or MRD-. Previously, patients had to be MRD+. Dose is 10 mg adjuvant CpG DNA and 4.9 mg 7P peptide. Oligometastatic disease was eligible before, now it is not. This Phase 2 cohort is less sick than their previous patients.
I will note that the clinical data we have is from the Nature Medicine papers that describe adjuvant CpG DNA at the same dose as the phase 2. But, Nature Medicine results only gave 2P peptide fragment (i.e., 2 mKRAS fragments) versus the 7 peptide fragments that the phase 2 gives and the AMPLIFY-7P Phase 1 gave.
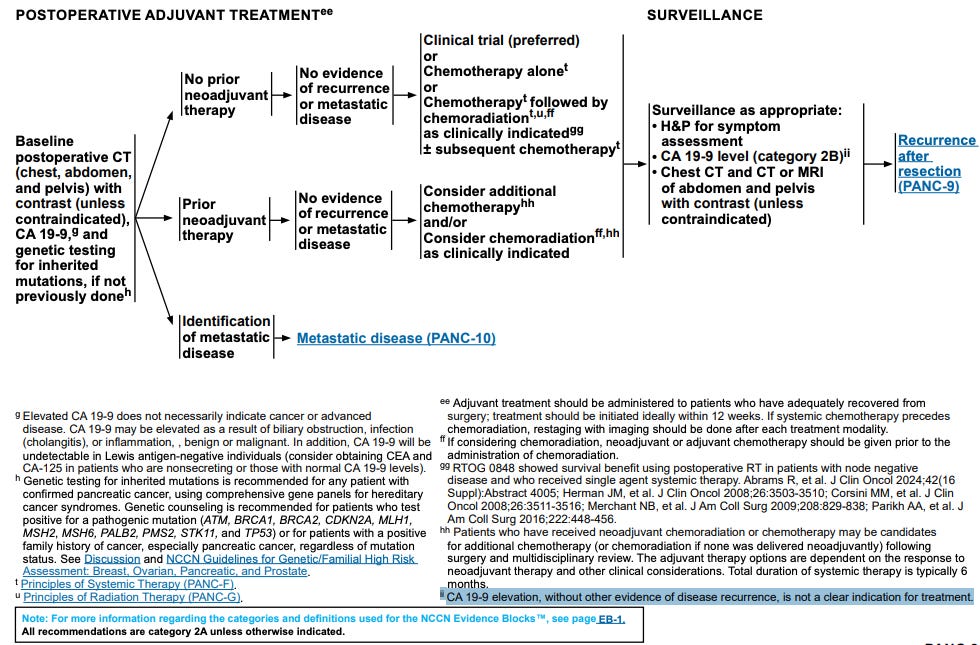
Observation is NOT the standard of care in MRD+ PDAC patients in the adjuvant setting. Chemotherapy, chemoradiation, or immunotherapy are the standard. Observation is not a good comparator. Even MRD- patients should be treated, at least a fraction of them per NCCN guidelines (see below).
If most patients are receiving observation then the control arm is doing a great disservice to these patients and is also not a good comparator. It is hard for their treatment to be worse than true observation. Even if the treatment is placebo, placebo against observation probably wins nine times out of ten because placebo can often times make patients feel like they are getting a benefit relative to nothing being done. In summary, if observation really is true observation and not investigator’s choice of adjuvant therapy (or lack thereof), then this trial does not offer as any useful clinical information to assess ELI-002 7P.
Now, let us consider the scenario where observation means investigator’s choice in the Phase 2 ELTX trial. Eileen O’Reilly, the MSKCC GI oncologist, gave a KOL presentation webcast on ELTX’s IR site in June 2025. She presented the following slide:
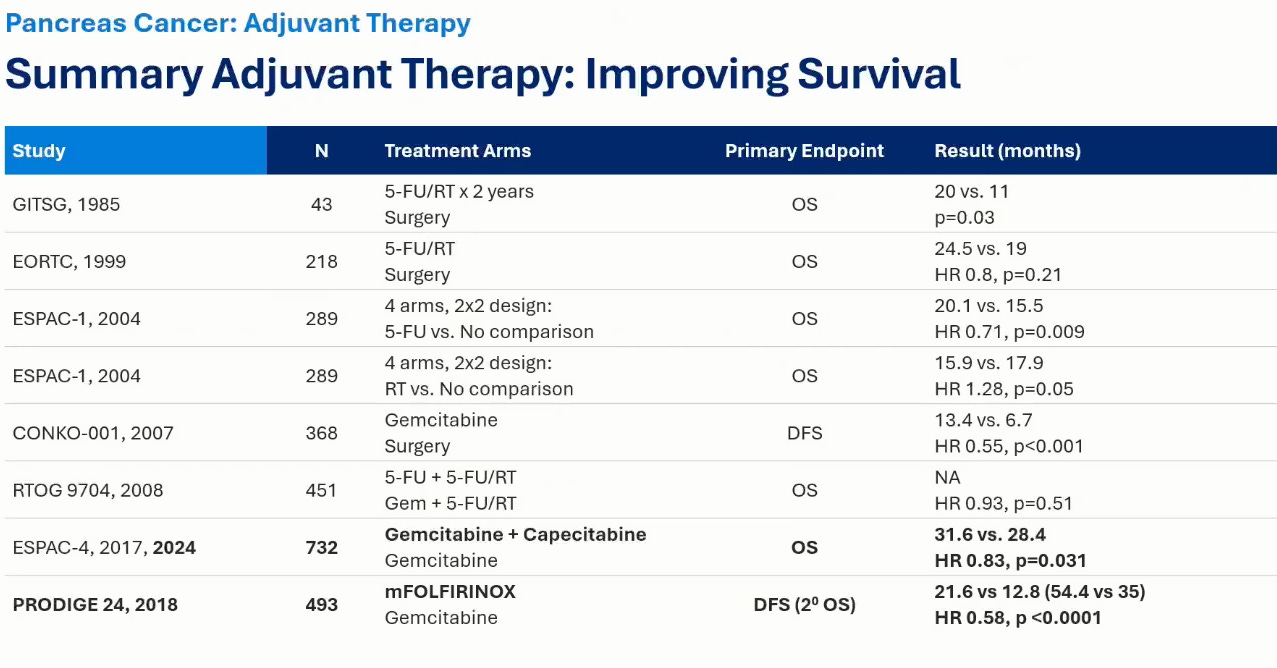
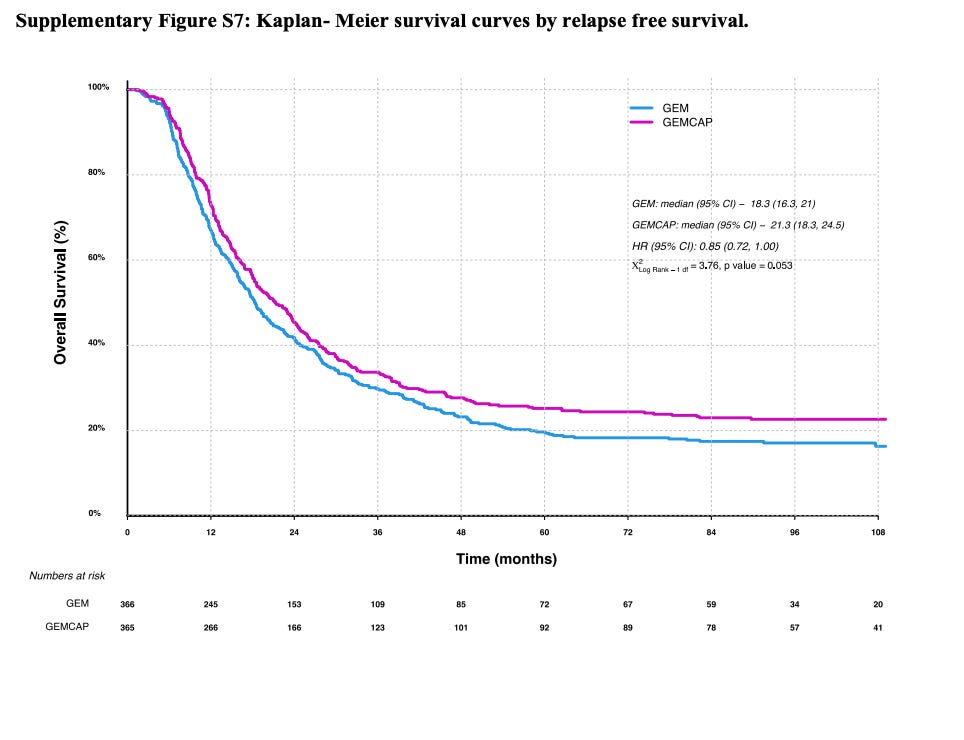
Notice that PRODIGE24 established modified FOLFIRINOX (mFOLFIRINOX) as the standard of care in the adjuvant PDAC setting. The modified in mFOLFIRINOX refers to a dosing schedule lower than traditional therapy that seeks to decrease side effects of chemotherapy (and it does do that). The line to meet is 21.6 months for disease-free survival per ELTX’s own KOL. That is also to say that if the observation arm has a mRFS substantially lower than 21.6, then we need to ask a lot more questions. We have evidence that another type of chemotherapy, gemcitabine, tried in ESPAC-4 produces a DFS of 18 months in adjuvant PDAC (see below). So, the absolute lowest mDFS that the observation arm should have in ELTX Phase 2 should be 18 months.
ELTX therapy is less toxic than chemotherapy but in order for it to be approved, in my opinion, it would have to at least be close to gemcitabine mRFS of 18 months if not pushing 21 months. Current ELTX PDAC mRFS is 15 months. And, that was with skimming the median and most patients getting subsequent therapy.
They are telling us that MRD+ PDAC has a median RFS of 5-6.4 months mOS of 17 months. Look above, their own KOL tells a different story.
We can look at the above slide and why it does not make sense by trying to validate the mOS numbers using ESPAC-4. ESPAC-IV showed margin positive PDAC (R1) on gemcitabine had median OS of 26 months (see below figure). This is way higher than the 17 month number they quote in their slides. This leads me to believe that they are reporting the observation no treatment adjuvant PDAC estimates. And, this makes me think that they are using true observation for most patients in the Ph2 trial.
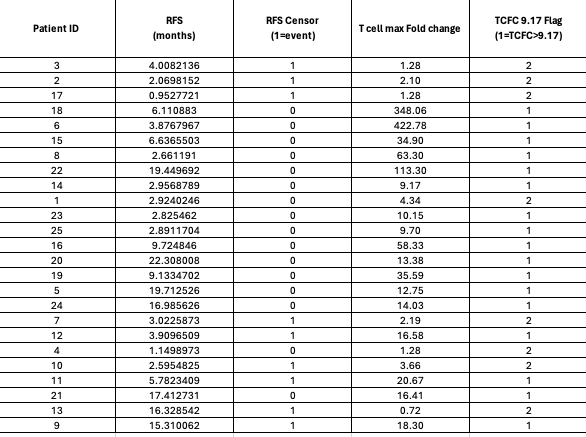
They say in their Phase 2, 80% have median T cell response above 9.5x which was basically the rate in the 7P Phase 1 data. Let’s investigate this claim. Figure 1 from 2024 Nature Medicine paper allows us to figure out which patient IDs had CRC and which had PDAC. CRC patients were ID 16, 19, 21, 13 and 7. Supplementary Source Data for Figure 1 from Nature Medicine 2025 paper gives us RFS in months and T cell max fold change for each patient ID. Granted this data is 2P (2 peptide) but the phase 2 is 7 peptides.
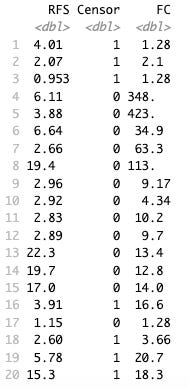
Here is the raw data of PDAC patients T cell response fold change (FC), whether they were censored or not, and RFS in months.
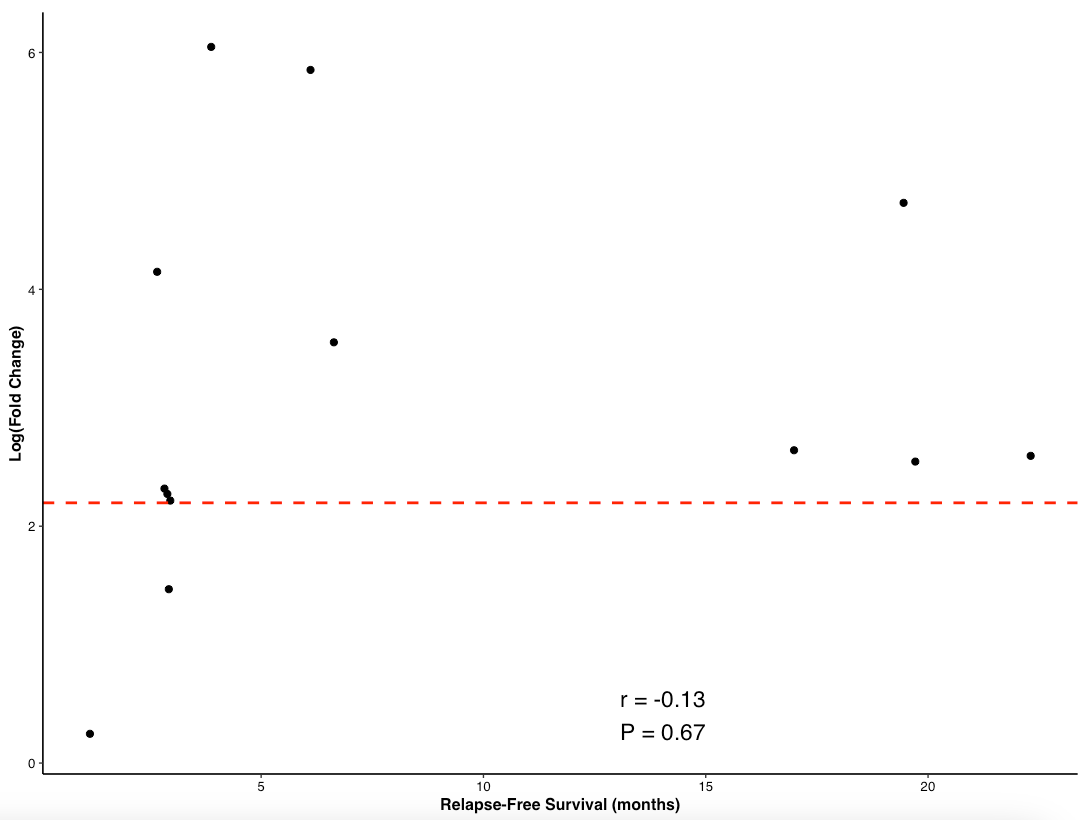
As you can see, there are a few patients with really high T cell responses so when we plot RFS by FC we can take log(FC) to make it interpretable. Here is a plot of non-censored RFS in months against T cell response.
Let us forget that this is an exploratory analysis and choose to use threshold as a predictor of outcomes. So, if a patient has a less than threshold response, they will almost surely have an RFS < 5 months. If a patient has above threshold response, they have a 50-50 shot of 5 month RFS or 18 months RFS. We know that in Phase 2 data, 80% have T cell response > threshold. That means 20% do not and they will have mRFS of ~5 months. The 80% are split between 5 months and 18 months. The median will be 12 months-ish. So 0.8*12 + 0.2*5 = 11 month mRFS is my guess. Of course, this is based only on MRD+ patients and on principle, MRD- patients should have longer survival but I can’t forecast that with the data at hand. This is higher than the 5-6.4m MRD+ PDAC comparison they put forth in their deck, but as mentioned above, this numebr is not the right comparison. 11 months is lower than the 21.6 months of PRODIGE trial shown above.
It is too hard to ascertain whether there treatment meaningfully shifts outcomes in the adjuvant setting for mKRAS PDAC. And I lean to there being no benefit here. There is much excitement in the PDAC space now with KRAS therapy (RVMD, VSTM, IMRX) and adjuvant vaccines are, from a biological perspective, incredibly cool.
Elicio has 22M in cash as of June 30, 2025 and expects to be able to fund operations into Q1 2026. This Q4 readout of Phase 2 disease free survival is a make-or-break event. They will raise cash if positive else I expect the stock to trade at cash after. This reminds me of the cardiff oncology readout earlier this year.
Thank you to Adu Subramanian for comments on the draft. View here only represent my thoughts.