Clascoterone is not a cure for baldness
Comments on their reported statistics and a comparison to finasteride
Cosmo Pharmaceuticals (OTCMKTS: CMOPF) recently released a press release from their two phase 3 studies of clascoterone in male pattern hair loss. Here is the clipping and the full link can be found here.
The 539% number has been quoted a lot on social media. Cosmo has not found the cure for male pattern hair loss. The 539% only considers one of the two trials they ran. And, both trials are identical with respect to what treatments they gave, how the trial was designed and which types of patients they enrolled. Sample sizes are roughly the same, assuming equal dropout rate in each study1, so the true measure that should be quoted is the average of 539% and 168% or 353%.
These two numbers being so different from one another suggest that the trials actually enrolled markedly different patients. This was not on purpose as you can read from their clinicaltrials.gov page for both trials. The clinics enrolling were different, so perhaps patients with more severe male pattern hair loss were enrolled in the 168% study or there was uneven dropout between groups which juiced the numbers in one group. It’s hard to say what exactly was different, we just need to wait for them to present the results. Let’s just pretend 353% is the proper goalpost to compare to for now with the understanding that the pooled number is probably lower.
First, what exactly is the metric they are reporting? 539% relative improvement over placebo and 168% relative improvement over placebo means what? Their primary endpoint is change from baseline to month 6 in non-vellus target area hair count. So, each patient gets a value D = {hair count at month 6} - {hair count at baseline} for the target area on the scalp defined at baseline. Now, take the mean of all the D values for the patients in the placebo group. Then, take the mean of all the D value for the patients in the treatment group. So, maybe in the treatment group we had an average of 40 hairs extra grow. And, in the placebo group we had an average of 8 hairs extra grow. Then, we take the improvement over placebo. Specifically, we now do (40-8)/8 = 4. Now, express it as a percentage, 4 * 100 = 400%. Ta da, 400% relative improvement over placebo. In obscures the actual effect size of how many hairs grew back.
Note that if the placebo group had a mean D value < 0 meaning they lost hairs, then the relative improvement metric cannot be calculated. As far as I can tell, the relative improvement metric was not pre-specified and certainly not present in any materials of theirs I can find online. What was pre-specified was the change from baseline to month 6 in treatment group relative to placebo. In the numerical example above, that would be 40 - 8 = 32 hairs. There are two reasons management would not report this number: 1) the number is so small and lower than any of the other trials out there that explicitly measure this (e.g., finasteride trials), 2) it can confuse the lay audience of non-scientists / hair people. If you read the hair literature, reporting the change from baseline to month 6 in both groups is standard so anyone investing in this field would get it.
There is also an ERROR in their reporting. They quote a 539% improvement relative to placebo. Then, in their fine print on the same press release, they write “5.39x relative improvement in TAHC versus vehicle“. A 2x improvement is a 100% increase. So a 5.39x improvement is a 439% increase. A 539% improvement is a 6.39x increase. Which is it! Here is the screenshot:
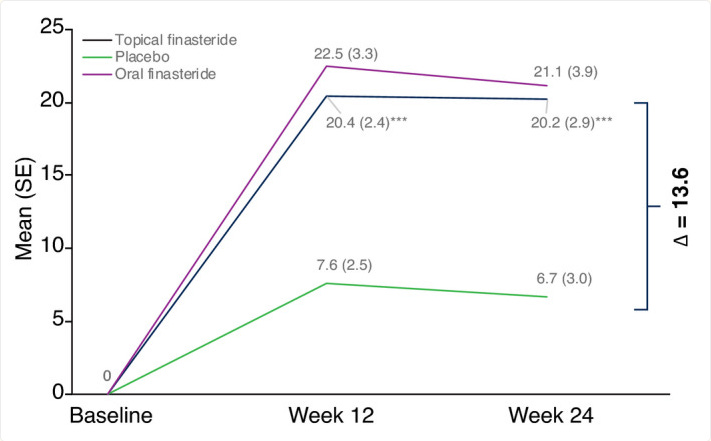
Topical finasteride is the best comparison to topical clascoterone. So, how does finasteride perform on the same endpoint in a very similar trial population that COSMO measured for clascoterone?
Roughly a (20.2 - 6.7) / 6.7 = 200% improvement relative to placebo at 6 months with N = 105 in topical and N = 97 in placebo. Depending on how you interpret the error in their press release, Cosmo either has a 353% improvement OR a 253% improvement ( 5.39x improvement = 439% and 1.68x improvement = 68%). Fairly similar. Clascoterone (Cosmo’s drug) is clearly doing something, but not curing baldness levels of something. And, one more thing to note is that the finasteride trial was only once daily application whereas the clascoterone was twice daily. The dose makes the effect (and side effect) with these sorts of medications.
Clascoterone (brand name Winlevi) is an FDA approved drug for acne vulgaris. The acne trials Cosmo ran showed that it was generally safe. 7-12% report redness, itching, and scaling. Hyperkalemia and HPA axis suppression could occur but rare. However, the acne FDA label is for 1% whereas the SCALP1/2 hair loss trials tried 5%. Dose makes the poison and we should expect greater side effects in SCALP1/2. With sufficient topical usage, the drug will enter systemic circulation. We know this is true because cases of hyperkalemia and HPA axis suppression occurred which would be impossible with topical-only clascoterone penetration. At 5% applied twice daily indefinitely, patients will see systemic effects. This makes the following statement an overstep:
“clascoterone 5% topical solution is the first topical androgen receptor inhibitor designed to target the biological root cause of male-pattern hair loss without the risks associated with oral therapies.”
This builds into the next point of mechanism of action. Clascoterone is an androgen receptor inhibitor. In the hair follicles, it prevents dihydrotestosterone (DHT) from binding to the androgen receptor and triggering hair-destroying pathways. Finasteride is a 5-alpha-reductase inhibitor. It inhibits type II 5-alpha-reductase which converts testosterone to DHT. Lowers amount of DHT available nd therefore limits the ability of DHT to bind AR in the hair follicles and elsewhere in the body. Long story short, clascoterone does basically the same thing as finasteride. Oral finasteride like oral clascoterone will have more off-target effects than their topical counterparts. Topical finasteride is basically equivalent in terms of on-target effect to clascoterone. We don’t have as much long term safety data on clascoterone as we do finasteride so it’s hard to forecast what may be reported with respect to libido and other sexual side effects.
Brief chart of the effect of testosterone and its metabolites:
In the SCALP1/2 trials, the exclusion criteria reads:
Topical scalp treatments for hair growth including minoxidil, Aminexil, hormone therapy, anti-androgens, or other agents that are known to affect hair growth within 12 weeks of Visit 2/Baseline”, “Platelet rich plasma (PRP) procedure on the scalp within 6 months of Visit 2/Baseline”, micro-needling, light or energy treatment, or hair transplants. Also, “Any 5 alpha reductase medications (i.e.: Finasteride [Propecia®, etc.], Dutasteride or similar products within 6 months of Visit 2/Baseline
So basically, the person can’t be taking anything else for at least 3 months if minoxidil and 6 months if finasteride. That’s a long washout period and basically ensures that therapy if it has any on-target effect will work. We know DHT blocking works to improve hair growth. If you do it by blocking the receptor that DHT binds OR decreasing DHT, it’s all the same thing.
In SCALP1/2, 726 + 726 patients in each. 2:1 tx to placebo in part 1 which is what the endpoint is based on. So, 242 for placebo in each trial and 484 to drug in each trial. ~30% dropout is expected even in the placebo arm of the finasteride trial that was what was observed. So, let’s say the analysis is based on 678 patients (339 in each arm on tx), and 340 patients (170 patients in each arm on pbo).