Predicting updated 6- and 9-month OS for Atebimetinib + mGNP ($IMRX)
Updated OS will be lower than most people think but that's okay
Immuneering ($IMRX) released phase 2a survival data of atebimetinib (deep cyclic MEKi) in combination with mGNP (modified regimen of gemcitabine/nab-paclitaxel) in first line metastatic pancreatic cancer in mid-June 2025. 9 months of median follow up will be presented Sep 25, 2025. We are going to try to guess at the upcoming updated 6 and 9 month overall survival.
Before, we do that, let’s remember management’s paraphrased words:
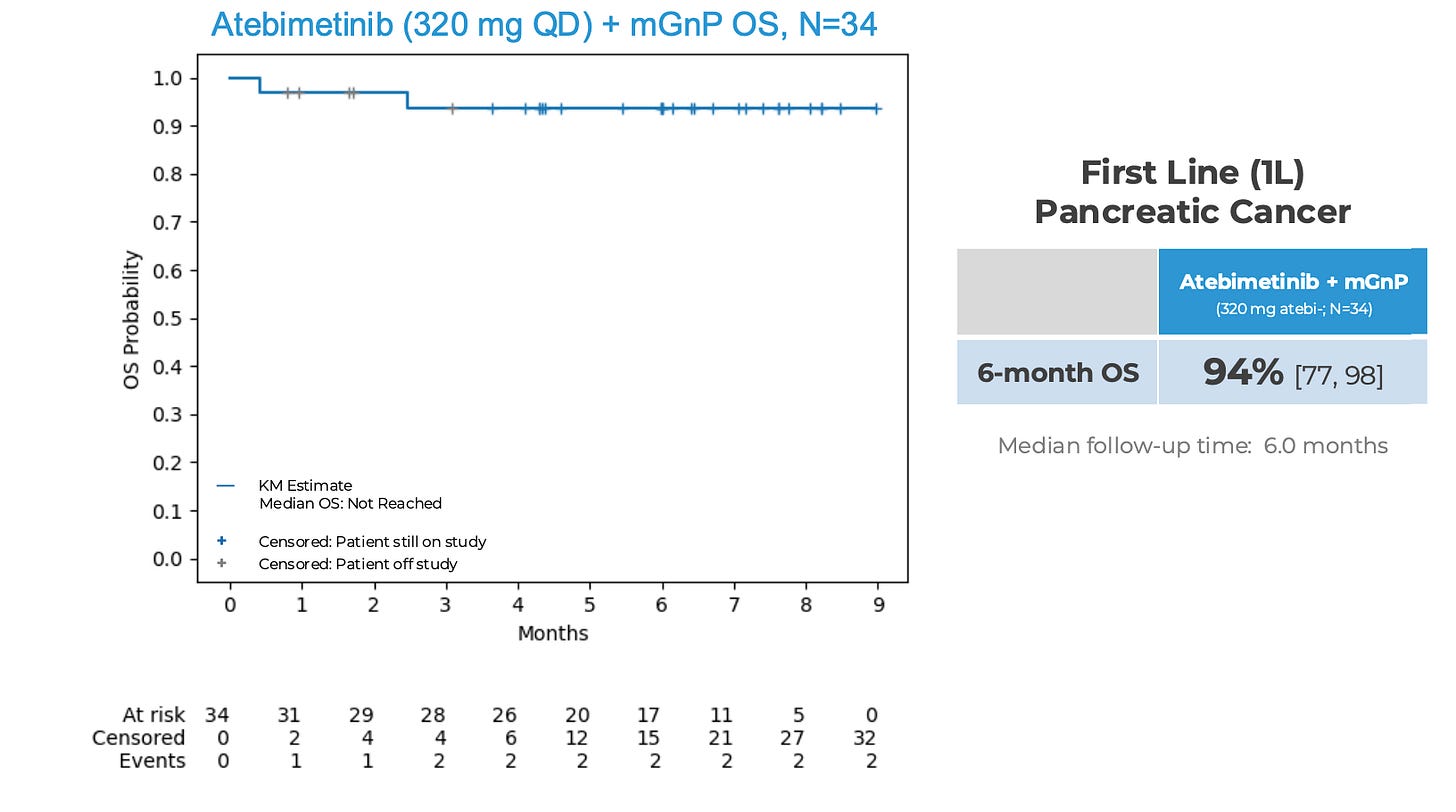
Less than half of patients treated with standard of care GNP in this setting survive 9 months. 94% OS and 72% PFS observed at 6 months in 1L PDAC treated with atebimetinib (320 mg QD PO) + mGNP (n = 34). Cutoff for data was May 26, 2025.
The clinical trial data for GNP revealed 6-month OS of 67% and dropped to 50% in 8.5 months. $IMRX’s study does not have a placebo arm so we have to look externally for a GNP bar. The bar is set at 67% 6-month OS and basically 50% 9 month OS.
Two things to point out:
When $IMRX released their original 6 month data in mid-June, they guided for additional data update in Q4 2025. Sep 25, 2025 is ahead of schedule.
PFS is often a leading indicator of OS because you will progress before you die and if follow up appointments are regular enough, we should catch progression before death. PFS leading OS is pretty solid in pancreatic cancer. See more in the footnote including some caveats1
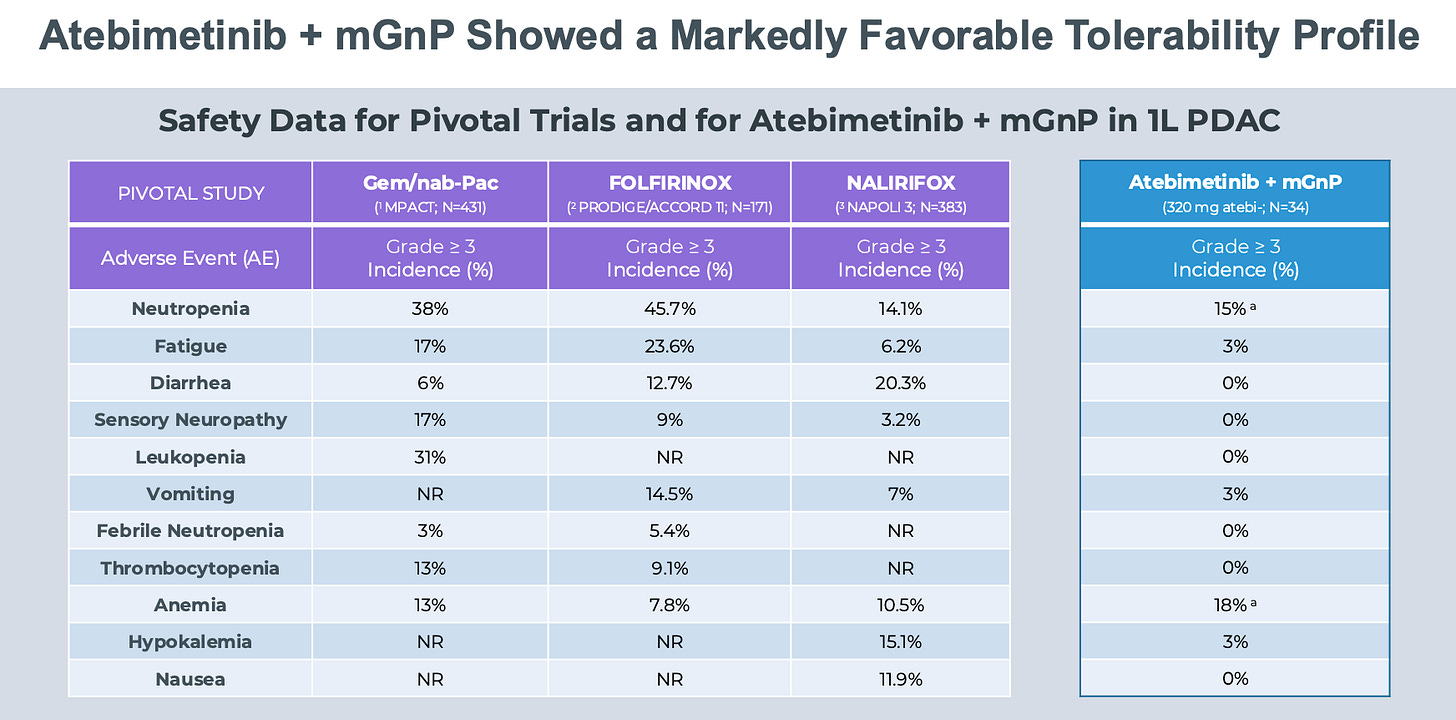
Part of the story is that Atebimetinib is an exceptionally well tolerated drug given previous high toxicity MEKi drugs. See below for their safety profile chart.
We need to compare apples to apples. Modified GNP is not the same as GNP (MPACT study). Modified GNP = 1000 mg/m2 of gem + 125 mg/m2 of nab-Pac days 1 & 15 q4 weeks whereas MPACT GNP = 125 mg/m2 of nab-Pac + 1000 mg/m2 of gem on days 1, 8, 15, 29, 36, and 43 after which all patients were administered treatment on days 1, 8, and 15 every 4 weeks. So GNP groups gets a lot more chemotherapy than mGNP. And chemotherapy AEs are drop blood counts (neutropenia, anemia, leukopenia), cause fatigue, and lead to GI side effects (diarrhea, vomiting, nausea). The safety profile of IMRX combination is likely due to less chemotherapy being given to patients. 2
Here are the IMRX survival analyses from their mid-June release.
Note that 5 patients were censored and off study early in follow up (grey ticks). And 10 more patients were censored before 6 months because they didn’t have follow up to that time yet (blue ticks). Last time, they presented data mid-June 2025 with data cutoff mid-May so it stands to reason that data until mid-August will be used in the mid-September update. Simply put, we will have 3 months more follow up for each patient in the study. The 10 patients with < 6 months follow up, will have 6 months follow up by Aug 25, 2025 (since last data was stopped May 2025), so we will know if they survived or not. Censoring early artificially props up short-term survival estimates until the data mature.
IMRX’s press release says N = 34 update with 9 month median follow up, so no new patients. They did mention expanding their cohort to 50, but we won’t be seeing that data here.
There were 5 patients that were censored early, within 3 months, who left the study. Why? Two reasons: 1) they sought curative or other treatment, 2) clinical progression necessitating a hospice admission or palliative non-treatment care. 2 is more likely than 1 for patients with metastatic unresectable pancreatic adenocarcinoma. It is my opinion that a conservative estimate would be that at least 3 of the 5 censored off-study patients died. If we factor that into our analysis, that drops the 6 month in the original analysis to around 85%. But, we won’t factor that in because that’s not fair to assume (even though I think its likely).
What might the 6 month OS be in the update? What might the 9 month OS be in the update?
6 month OS updated with additional 3 month follow up. Anyone with at least 3 months of follow up will be included here. Previously, we had 17 at risk at 6 months. Now, we will have 27.
What might the updated 6 month OS be? The current 6 months OS is defined as (1 - 1/34)*(1 - 1/29) = 0.937. If none of the 10 patients who previously had follow up ranging from 3-5 months did not die, the 6 month OS stays the same. If any one of them die, the OS estimate gets revised down.
Of the ten, conservatively, 3 might die before 6 months. Let’s assume they die at 5 months. If that happens the 6 month OS is now (1 - 1/34)*(1 - 1/29)*(1 - 3/27) = 0.83. If 4 or more deaths occur, the 6 month OS rapidly approaches the GNP 6 month OS of 0.67.
9 month OS updated with additional 3 month follow up. Anyone with at least 6 months follow up at the time of the first analysis will be included in the update and not censored. 17 patients had 6 months of follow up back then so we should expect 17 at risk for the 9 month OS presented Sep 25. The expression for 9 month OS is roughly 6-month OS * (1- K/17) where K is the number of deaths that occur in the 17 patients who will had follow up to at least 6 months in the old update and will have updated up to 9 months now.
What might the 9 month OS be?
Assume no deaths in the 10 patients with follow up from 3-6 months in the old update.
If K = 3, 9 month OS = 77%; K = 5, 9 month OS = 66%; K = 9, 9 month OS = 44%
Assume 3 deaths in the 10 patients with follow up from 3-6 months in the old update.
If K = 3, 9 month OS = 68%; K = 5, 9 month OS = 58%; K = 9, 9 month OS = 39%
Let’s be as generous as we can and assume zero new deaths until 6 months and only 3 deaths between 6-9 months. The OS is 77%. That’s the most generous version. I think we hover around 9 month OS of 65%. To be clear, 65% 9 month OS is higher than the ~50% OS of GNP at 9 months with less toxicities. So, its still a better option than current standard of care. Mizhuo says “9 month OS of 75% would represent excellent outcomes”. I don’t see that happening.
Of course, confidence intervals will be wide given small sample sizes. We also need to keep our eye on the number of censored off study patients and how many more we get of them in the update. OS numbers will not hit the level people are expecting in part because this is not a miracle drug (though I think its a good drug) and metastatic unresectable pancreatic cancer is an incredibly aggressive disease.
What should the valuation of $IMRX be and where might it shake out in the competitive landscape are two excellent questions I will cover in the next post.3
The discordance between early tumor-based end points and OS is bidirectional, and the US Food and Drug Administration (FDA) has evaluated multiple trials in which an OS advantage has been demonstrated without substantive improvements in PFS or ORR. Examples include recent approvals of immune checkpoint inhibitors for non–small cell lung cancer, head and neck squamous cell cancer, and melanoma. In several trials, statistically significant and clinically relevant improvements in OS have been demonstrated; however, minimal or no improvements in PFS or ORR have been observed in the same trial (Table 1).10-13 Unlike conventional cytotoxic drugs where the relationship between early end points and OS has been more consistently observed, the unique mechanism of action of the immune checkpoint inhibitors and other treatments that may alter tumor growth kinetics rather than solely act via direct cytotoxicity may account for this disconnect
From this paper
The reason you have less chemo-related side effects is that you are giving less chemo! Consider selumetinib (dual MEK1/2 inhibitor) tried alone or in combo with paclitaxel in metastatic uveal melanoma. Their MEKi only arm toxicities were pretty low which gives us reason to believe that IMRX MEKi is also pretty safe. But it’s not magically cutting the AE rate.
It’s fair to think of IMRX as a single asset single indication company for now. There are two planned phase 2 trials: 1) Lilly Olomorasib (G12Ci) + Abemetinib in locall advanced or metastatic KRAS G12C-mt NSCLC who have progressed on prior therapy, 2) Atebimetinib in combination with PD-L1i (Libtayo) in patients with NSCLC.