On halting the progression of frontotemporal dementia
In people with a pathogenic progranulin mutation
What would a reasonable bar for success look like given that we have no disease modifying therapies available to treat frontotemporal dementia due to a pathogenic progranulin mutation (FTD-GRN)? After a one-time minimally-invasive treatment, patients early in the disease course with FTD-GRN experience only age-related neurological changes.
FTD-GRN accounts for 10% of all frontotemporal dementia (FTD) cases and are caused by loss-of-function mutations in the progranulin gene. FTD-GRN is an autosomal dominant disease with 90%+ penetrance by age 70. Survival is six years from symptom onset. Cells in the CNS make progranulin which they then secrete into the extracellular space. From here, cells endocytose progranulin where they reside in lysosomes, are processed into granulin proteins, and help lysosomes function properly. In FTD-GRN, an extracellular progranulin deficiency leads to lysosomal dysfunction (reduced cathepsin D activity, impaired trafficking of prosaposin and decreased glucocerebrosidase activity). In microglial cells, progranulin deficiency prevents lysosomal clearance of myelin debris. In astrocytes, progranulin deficiency promotes profound synaptic degeneration. Progranulin-deficient neurons accumulate TDP-43, tau and phosphorylated alpha-synuclein. All together, these changes lead to the progressive loss of neurons in the frontal and temporal lobes.
FTD-GRN is similar in setup to Alzheimer’s disease (AD) and therefore, we can learn a lot from the trials and drugs in AD. EVOKE/EVOKE+ tried semaglutide in AD and there have been a number of trials of anti-amyloid beta (anti-AB) antibodies in AD. Semaglutide did not change the course of AD neurological functioning scores compared to placebo but a number of the anti-AB drugs did. They slowed the progression of AD by a small amount. The issue is these are IV drugs that require dosing at least every 4 weeks, and carry a decent risk of brain bleeds. The principle of the anti-AB drugs are that they attempt to intervene on the pathological process in AD (amyloid beta plaques) and seek to slow progression of disease. Very similar ideologically to the FTD-GRN goal. The issue is in execution with the anti-AB drugs: dosing frequency, route of administration, side effects, intervention too late in disease course, and limited ability to halt progression of disease.
However, if you offered a patient with AD the pure treatment benefit of anti-AB drugs (small help in cognitive scores) BUT it is now a one-time treatment and significantly safer, I think a lot of folks would take it. Anti-AB drugs were offered too late in the disease course in the AD trials (and carries many side effects). And, we can run into a similar problem with FTD-GRN. FTD-GRN may first start as a progranulin haploinsufficiency problem then, over time, progress into the buildup of other toxic byproducts in the brain which could continue damaging the brain independent of the original progranulin issue (credit to private conversations with medstudentinvst). It’s like a runaway train that’s off track and heading straight for a mountain. Nobody cares that the reason the train went off track was because of a malfunctioning screw. Replacing that screw at this point does nothing. From the AD space, we should also remember that treating patients as early as possible in the disease course is best. Therapies in FTD-GRN and AD cannot reverse damage or regrow neurons or their connections. We can only hope, at best, to preserve remaining function. So it stands to reason that the earlier in the disease course we intervene, the better.
Now that we have some guiding principles, let’s evaluate the FTD-GRN therapy space: 1) Prevail-Lilly, 2) Passage Bio, 3) Alector, 4) AviadioBio
1) Prevail-Lilly - AAV9-GRN gene therapy
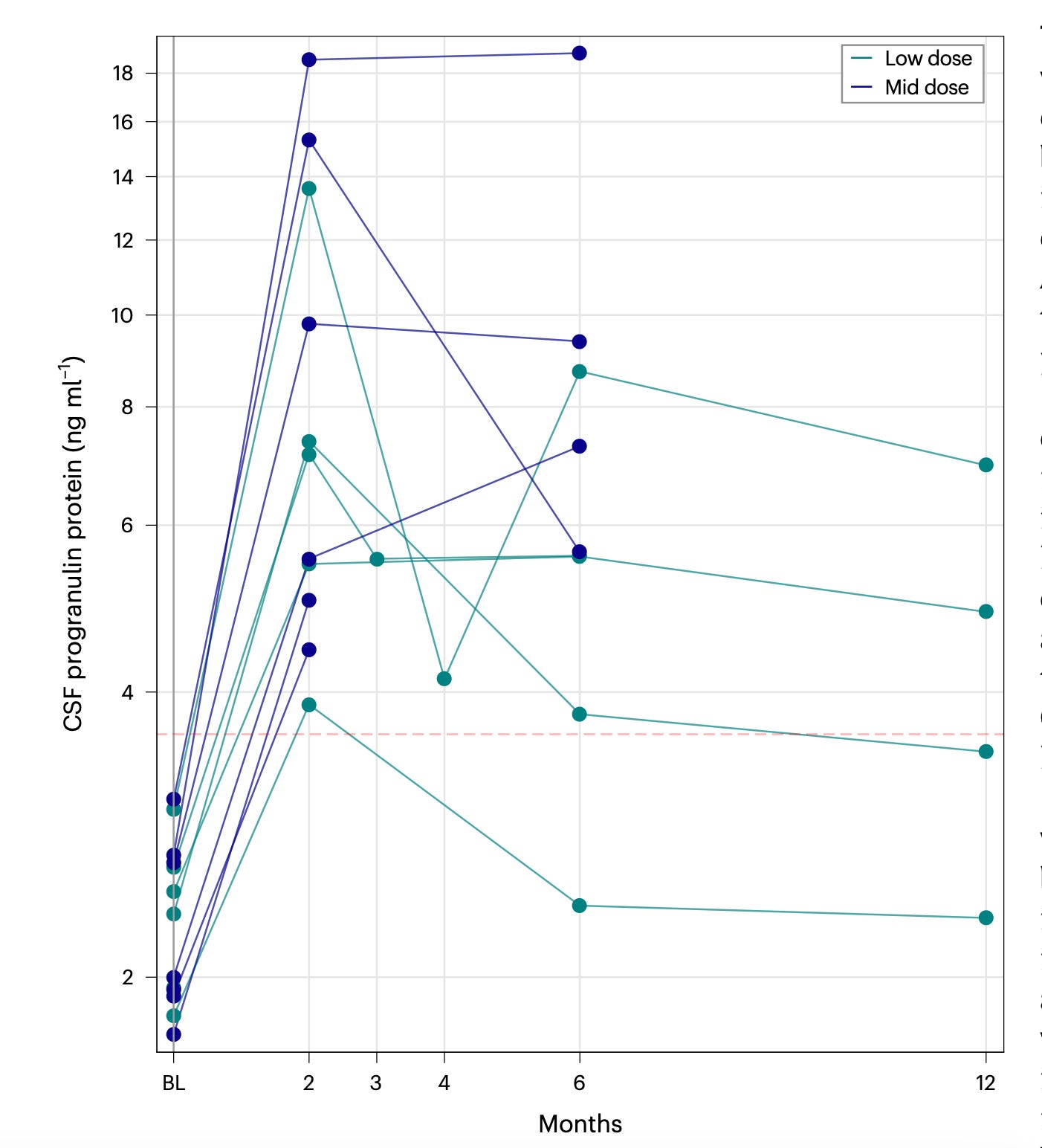
Prevail Therapeutics is trialing an intracisterna magna injection1 of AAV9-GRN for treating FTD-GRN. They were bought for 880M + 160M CVR in 2020 based on early phase 1/2 trial data. In a 2024 paper, they reported low and mid dose data where mid dose was 2x low dose. CSF progranulin levels over time are shown below:
Normal CSF progranulin levels range from 3-8 ng/mL with variance in the range depending on who you ask and how you measure (we’re talking about NANOgrams). For 4 patients with 6 month mid-dose data, median CSF progranulin level was 8 ng/mL and low-dose was 6 ng/mL. Progranulin reached upper limit of normal (ULN) or 1.5x ULN with a clear trend of a peak in progranulin at 2 months followed by ~50% declines from the 2 month peak over the next 10 months.
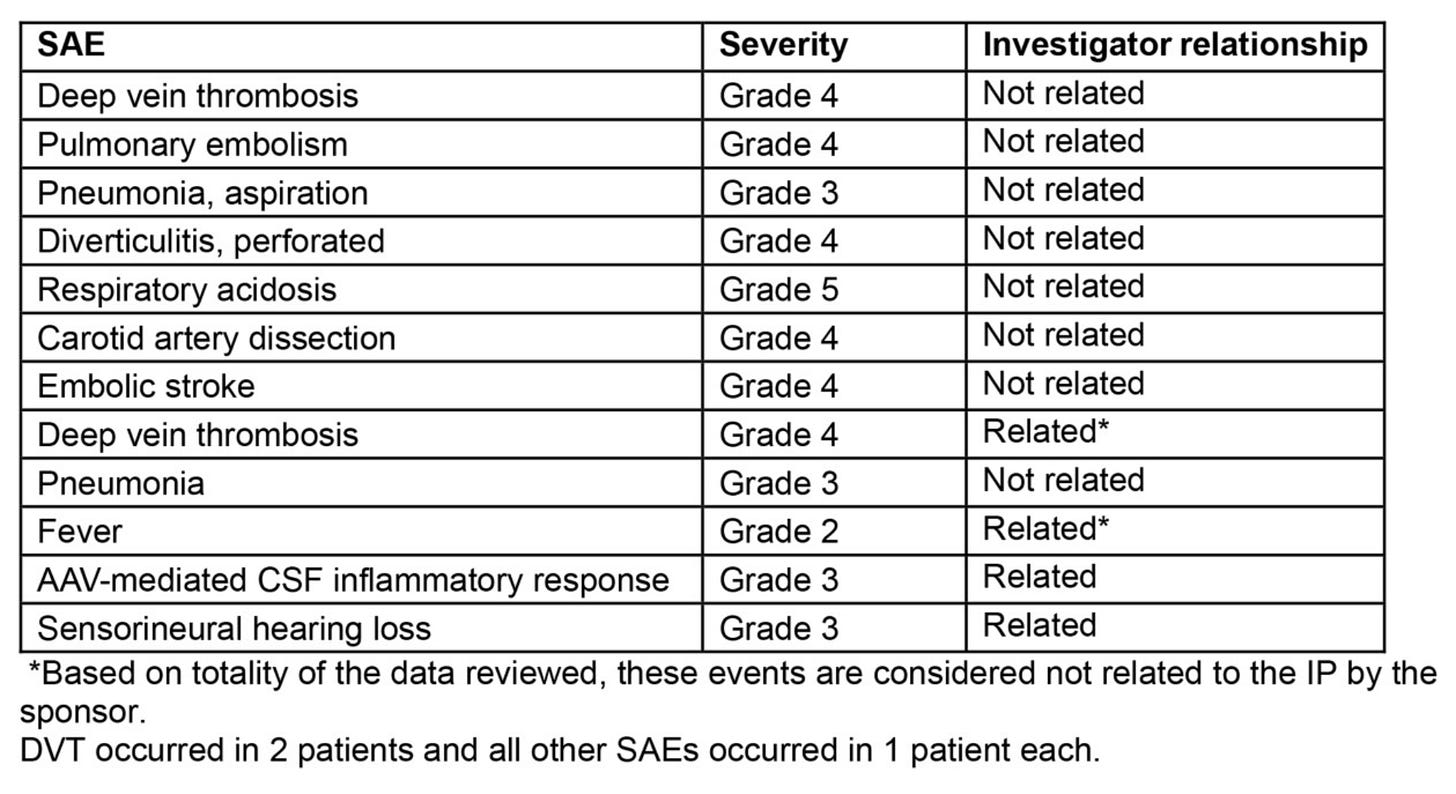
Adverse events from the paper listed above. Three thrombotic events within 2 months noted. Risk factors included concurrent sirolimus and croticosteroids use and BMI high and immbolity per paper. Further, from looking at the table above, regardless of how they judge the relationship, we see 3 DVT/PE events, and at least 3 pure AAV immune-related conditions (fever, CSF inalmmatory response, sensorineural hearing loss). A good FTD-GRN therapy would not have these side effects.
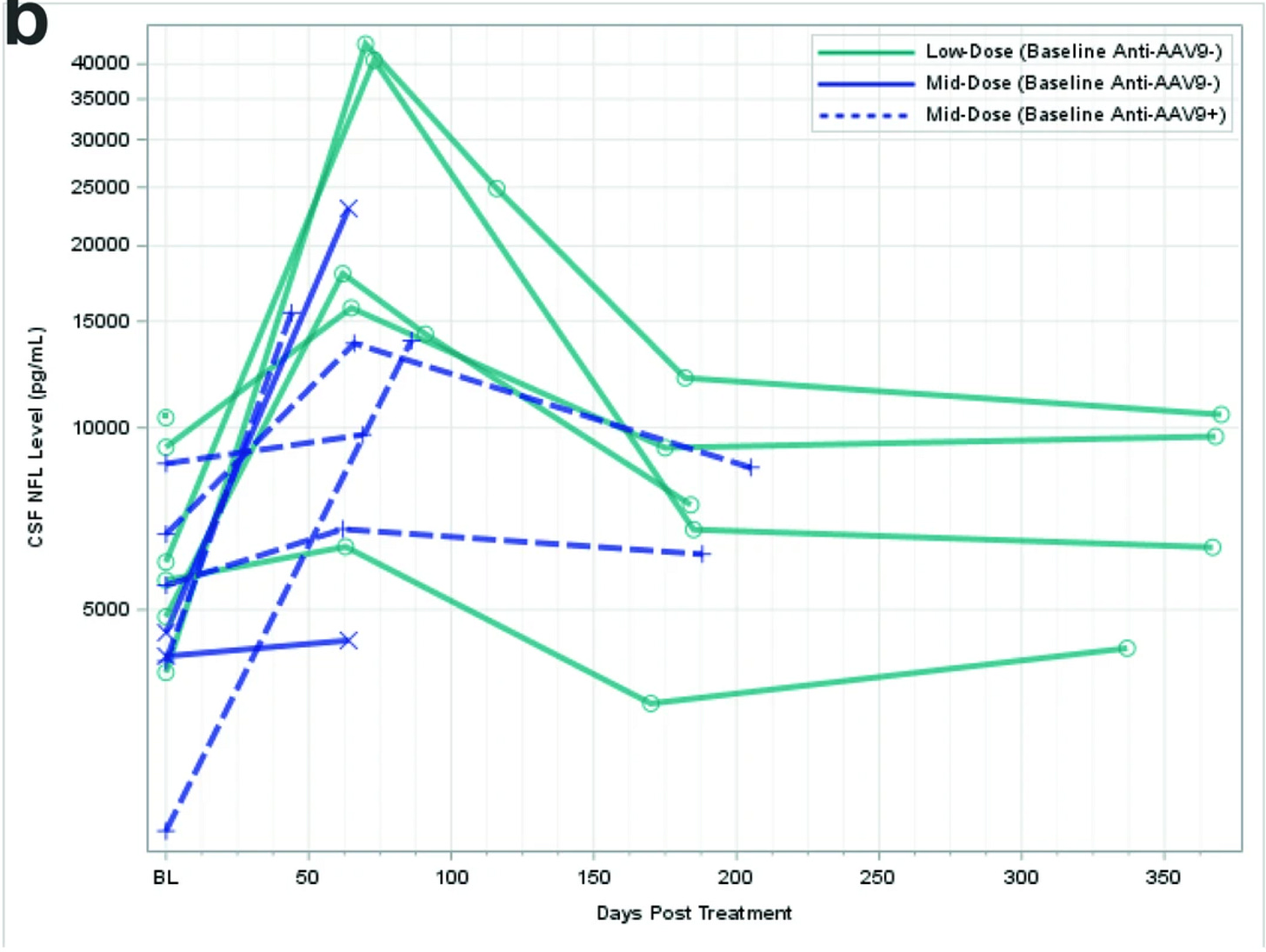
CSF NfL is a sensitive but not specific marker of neurodegeneration. And, we can see in the above graphs a clear spike in CSF NfL at 2 months followed by a retreat over the following 10 months. At ~6 months, here are the rough CSF NfL changes: low dose, 6000 —> 3000; mid-dose 5500 —> 6000 (anti-aav9+); low dose, 6000 —> 6500, low dose 5000 —> 7000, mid-dose (anti-aav9+) 6000 —> 8000, low dose 9000 —> 9000, low dose 5000 —> 12000. Very rough estimates but directionally, we see that at 6 months NfL is 10%+ higher at least. Not good.
The authors propose that NfL transiently increased in CSF at both dose levels because of a secondary inflammatory response. NfL trends mirrored progranulin levels: 2 month peak followed by drop. Efficacy comes with adverse effects for Prevail.
But, what about their cognitive scores? CDR global was 1.42 (SD = 0.67) and 1.57 (0.53) in low (n = 6) and mid (n = 7) dose at baseline. CDR sum of boxes was 8.08 (5.07) and 9.42 (3.37), respectively. At month 6, the sum of boxes total scores increased compared to baseline in the low-dose and mid-dose cohorts by, respectively, 2.30 ± 2.61 and 3.90 ± 2.68 (mean ± s.d.). At month 12, in the low-dose cohort, the sum of boxes increased by 4.70 ± 3.23. The authors conclude that this is in line with the ranges of natural history FTD-GRN cohorts. Said another way, treatment does not confer materially different trajectories than that of untreated FTD-GRN patients. Unfortunate.
2) Passage Bio - AAV1-GRN gene therapy
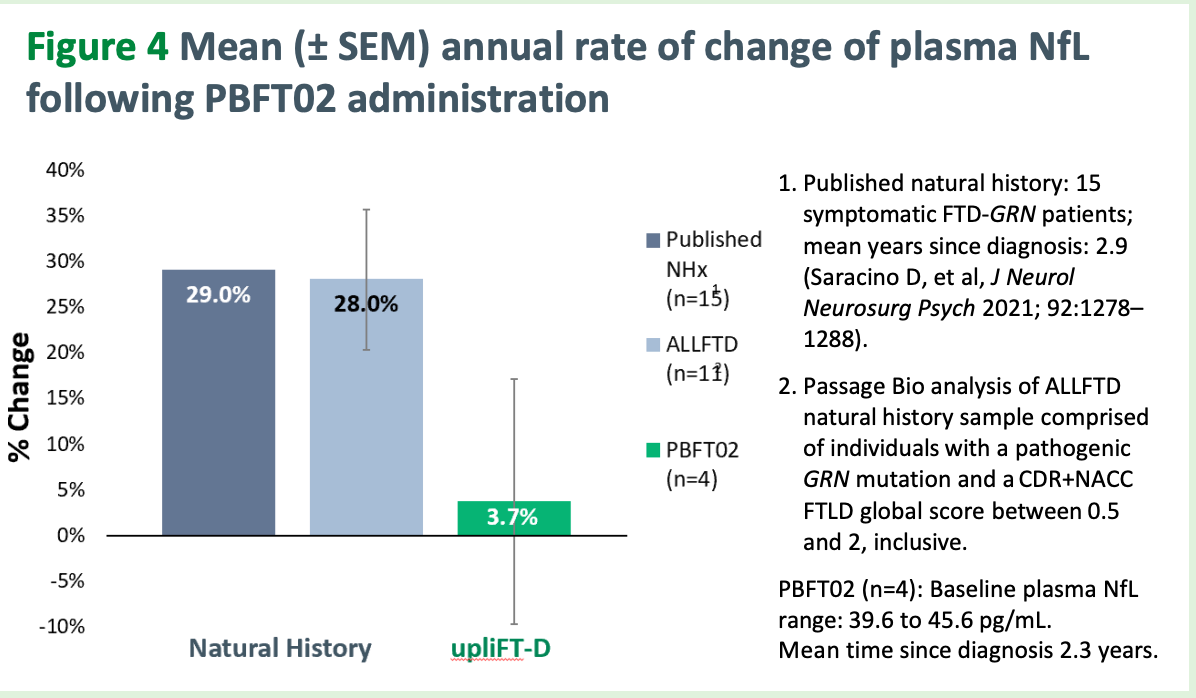
Passage is trialing an AAV1-GRN gene therapy via intracisterna magna injection. The belief here, as it is with Prevail, is to replace progranulin in the CNS extracellular space such that neurons and glial cells can endocytose it, restore normal function, and prevent the progression of disease. As their CEO Will Chou said, “we do not replace neurons so we have to intervene early, we preserve”.
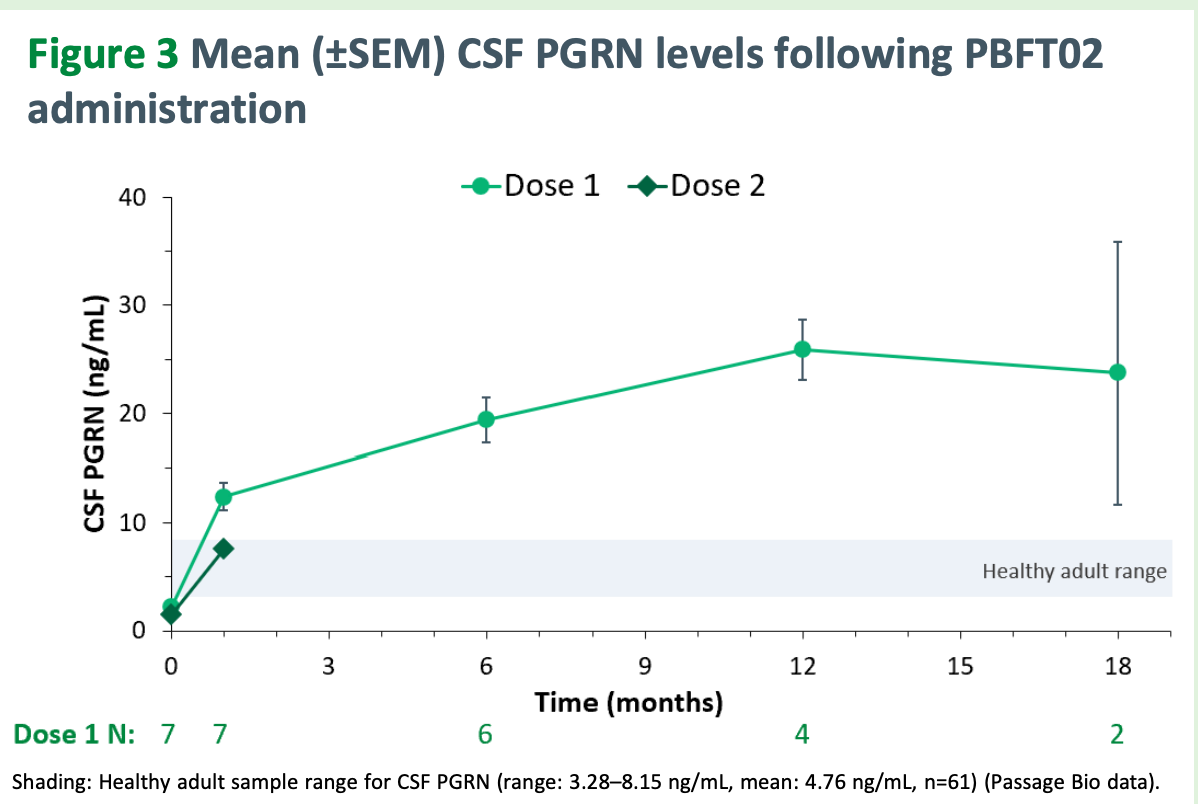
Normal range is 3-8 ng/mL of progranulin (PGRN). They are aiming to get mean CSF-PGRN at or above 8 per Will’s comments in early Nov 2025 on a Guggenheim webcast. Here is their most recent data from their ongoing phase 1/2 trial. Note that dose 2 is 50% dose 1 and the data for dose 2 is only for 1 patient. More data for dose 2 to come in the coming months.
Immediately, we notice the dose 1 PGRN levels are much higher than the Prevail ones and the PGRN levels go up consistently over time. For these 8 patients, CDR Sum of Boxes is mean 10.3 (5-17 range) with 50% CDR global 1 and 50% CDR global 2 with mean disease duration 2.9 years.
Passage’s NfL data are much better than Prevail’s. Jury is still out on whether this translates to cognitive scores declining at a slower rate than untreated patients.
Critically, no systemic inflammatory response or CNS inflammatory response observed. No complications with ICM administration. And, like Prevail, low dose anticoagulation will be given with treatment to prevent DVT. After the first patient in the first dose developed an asymptomatic venous sinus thrombosis and hepatotoxicity, the immunosupression regimen was updated to be 3d IV methylpred followed by methylpred PO.
3) Alector - anti-sortillin antibody
Alector trialed an anti-sortillin antibody for FTD-GRN given IV every 4 weeks. IV dosing every 4 weeks is much more of a burden for patients than a one time 1 hour procedure.
Sortillin is a cell surface receptor that allows for the uptake and subsequent lysosomal degradation of progranulin. Alector reasoned that inhibiting sortillin may be a viable strategy to preserve progranulin. Inhibit an inhibitor. This is an indirect way of approaching the primary problem of progranulin deficiency. But, there are two problems: 1) intralysosomal progranulin deficiency is the issue in FTD-GRN NOT extracellular, and 2) lysosomal processing of progranulin by sortillin, prosaposin, or other pathways is necessary for normal lysosome function.
Measuring extracellular progranulin is a proxy for the intracellular level ONLY when the pathways for endocytosis are not messed with. Otherwise, these the concentration in one compartment does not need to be a marker for the other. These were comments that were echoed by Passage CEO Will Chou too.
Let’s review the clinical data from their trials. Here is their INFRONT-2 open label data:
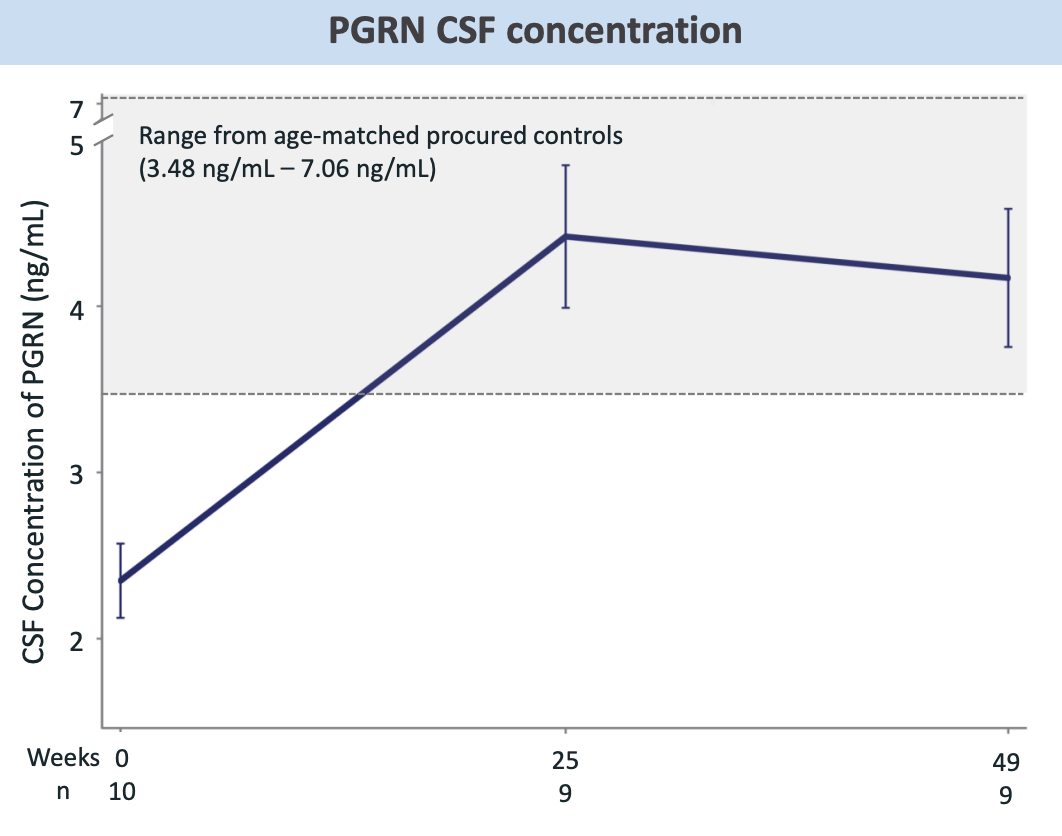
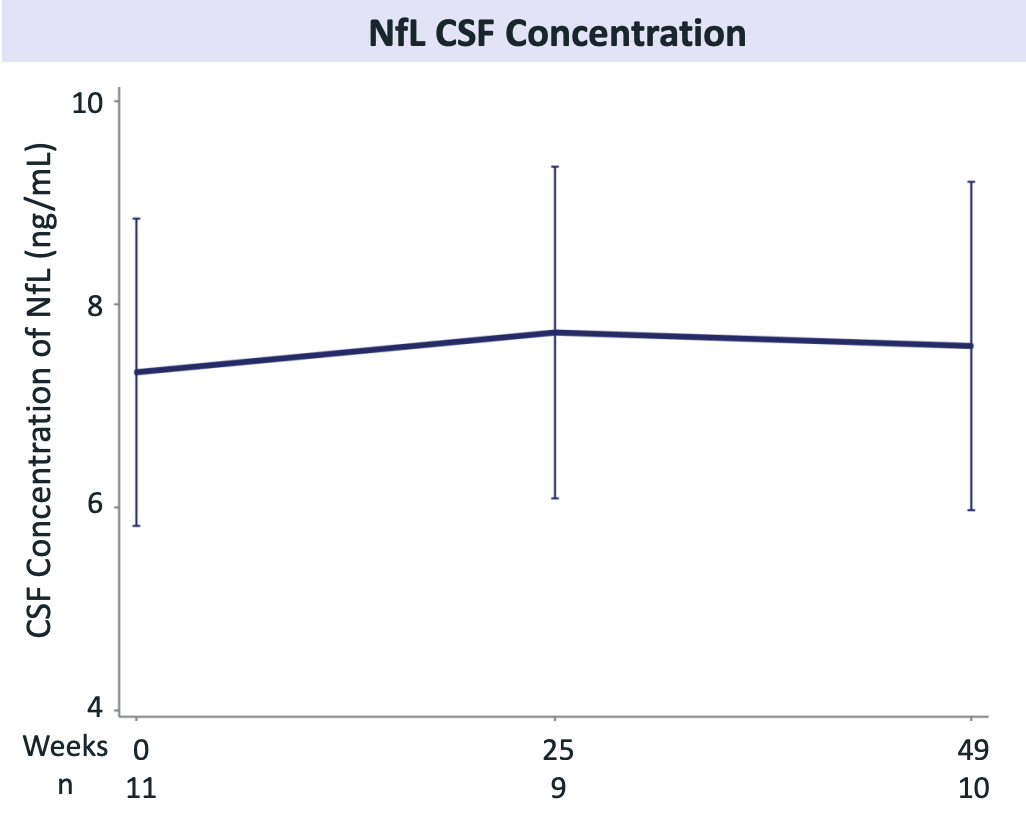
Notice that at maximum, CSF PGRN is not anywhere near the Passage CSF PGRN. Alector’s CSF PGRN data sits squarely in the 3.5-5 ng/mL range which is far lower than the AAV approaches. NfL CSF concentration in FTD-GRN cohort is stable over time which is certainly better than increases over time and maybe even in line with age-related changes.
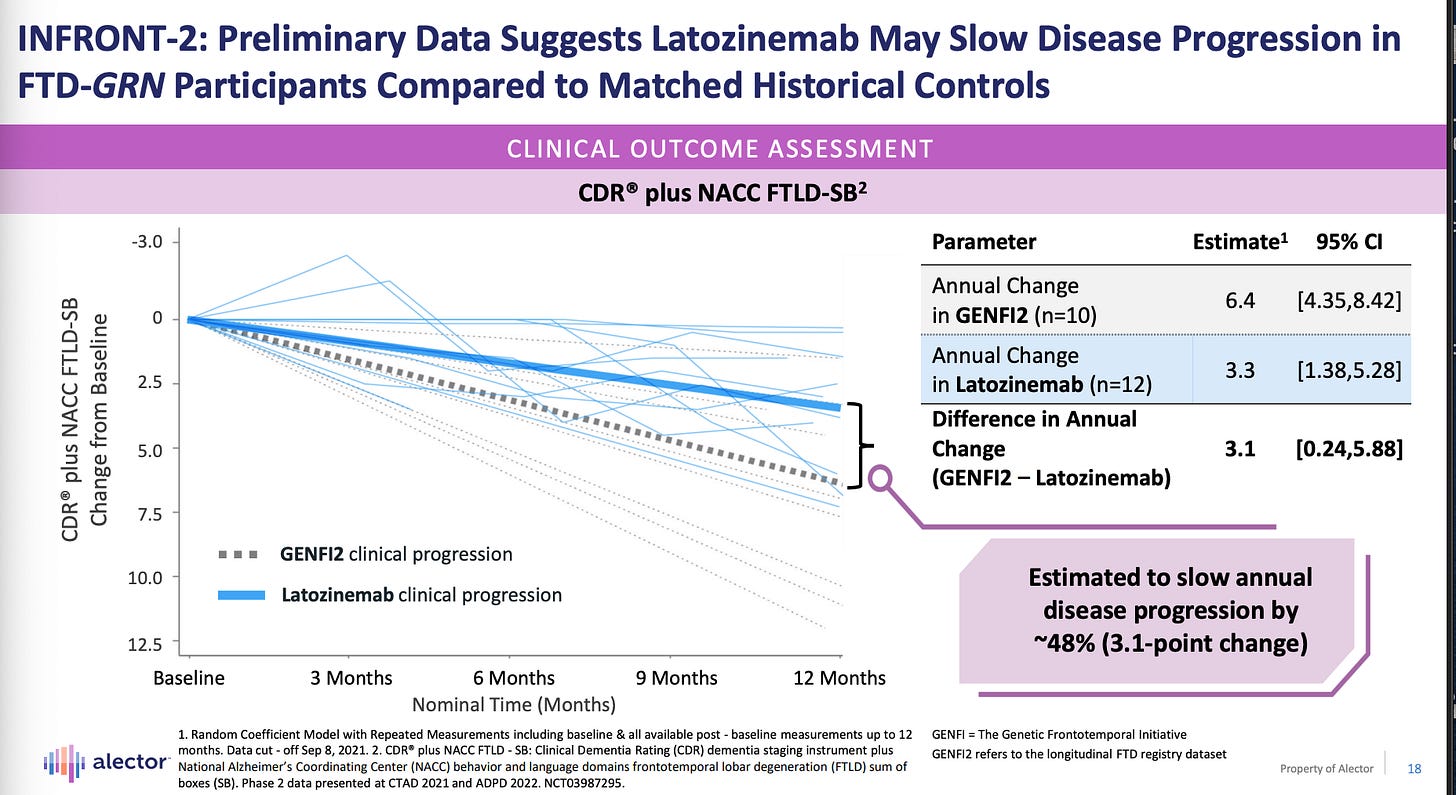
Here are the cognitive outcomes trends:
We see that CDR-SB + NACC FTLD-SB trajectories are different between natural history data and open-label INFRONT-2 data. The 95% confidence intervals of the INFRONT-2 and natural history data overlap though.
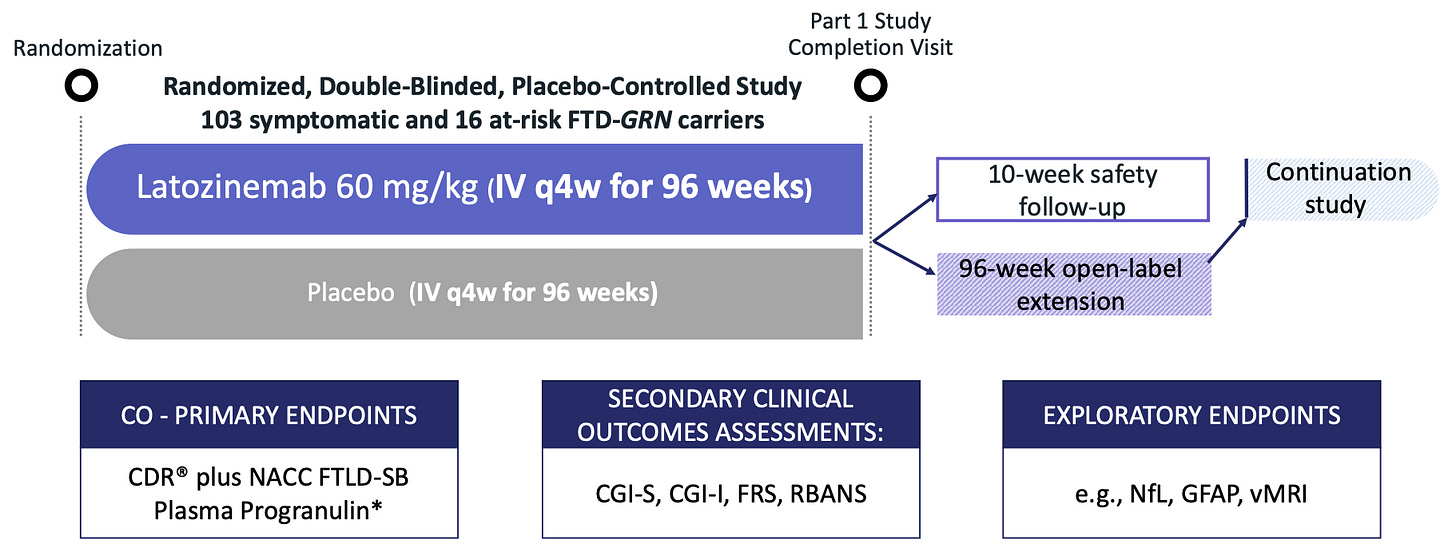
INFRONT-2 finished end of 2021 (12 mo period), then Alector started INFRONT-3 that took until Q4 2025 to finish and read out. Here is the phase 3 trial structure which had a placebo group.
“In the 96-week, double-blind INFRONT-3 trial, latozinemab did not meet the clinical co-primary endpoint of slowing FTD-GRN progression. Although treatment resulted in a statistically significant effect on the biomarker co-primary endpoint of plasma progranulin (PGRN) concentrations, the secondary and exploratory endpoints, such as fluid biomarkers and volumetric magnetic resonance imaging (vMRI), demonstrated no treatment-related effects on FTD-GRN”.
We must await their presentation, but while we do there is one thing to note from the INFRONT-2 and INFRONT-3 inclusion/exclusion criteria2. The phase 2 allowed only for “good physical health” and “no clinically significant findings from medical history, physical exam, lab tests, ECGs or vital signs”. That enriches for people who are healthier and more likely to 1) be able to handle a drug with side effects, 2) come every 4 weeks for therapy, 3) more likely to respond because they have a lot of physiological reserve and clearly, their fatal condition has not progressed too far. This is a big change in my opinion.
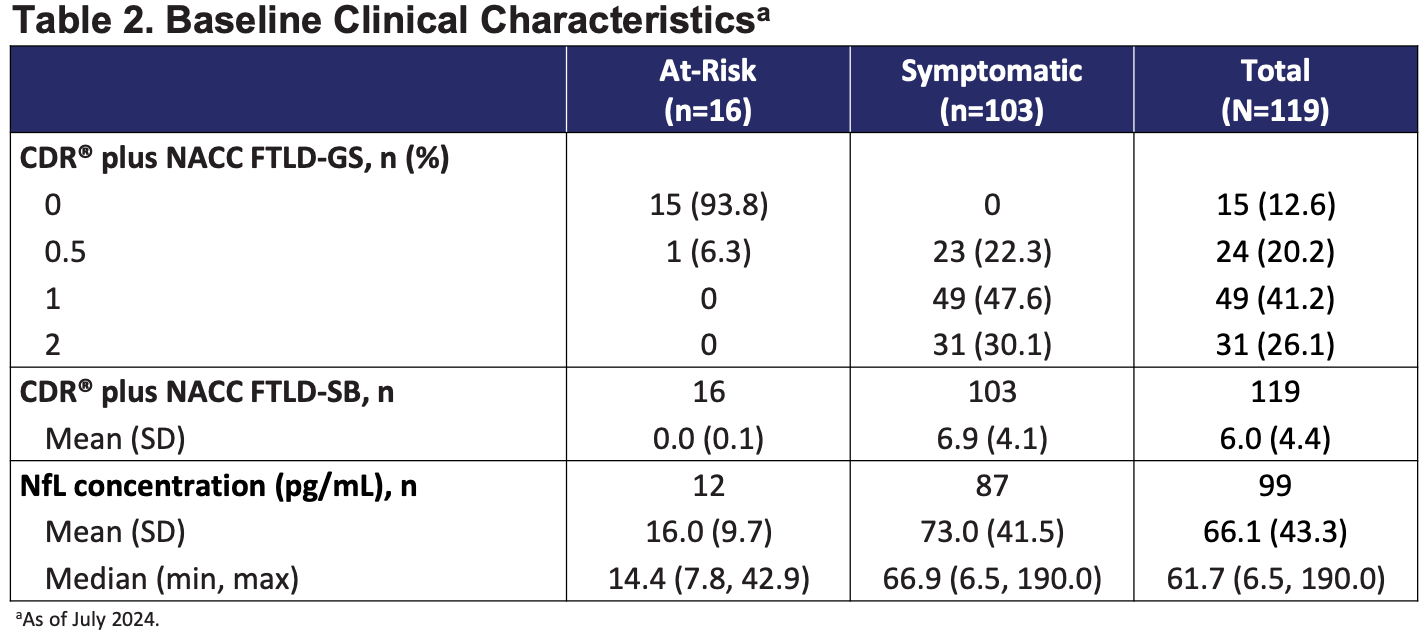
I should note that the phase 3 enrolled 16 of 119 patients with CDR + NACC FTLD of 0 which means these folks had no impairments as measured by the survey instrument. These patients, if untreated, will have better cognitive scores and NfL levels at 12 months compared to CDR+NACC > 0 patients.
We know the drug does not boost extracellular PGRN levels much and we know that this drug blocks one of the two main pathways of progranulin endocytosis. Therefore, this drug can’t boost intracellular levels much and these CDR+NACC FTLD = 0 patients on-treatment will experience a steady march of their disease and NfL patterns consistent with their off-treatment counterparts. Reasons the Alector Phase 3 failed: trial population changing to Alector’s detriment, drug producing a CSF PGRN of only 4 ng/ml, and issue that blocking sortillin impairs intracellular PGRN activity. Alector walked so others could run.
4) AviadioBio/Astellas - AAV9-GRN gene therapy
Intrathalamic AAV9-GRN gene therapy infusion currently in phase 1/2 trials (ASPIRE-FTD). They cite “biodistribution issues with approaches including systemic injection and cisternal magna injection”. And, I think the intrathalamic infusion risks outweigh the benefits.3 The only real differentiation between Aviado and Prevail is the route of administration and that Prevail has a multi-year head start. Maybe intrathalamic is the best but that’s not what I think.
They seem to be having a tougher time recruiting. Started trial 8/30/2023 and as of June 2025, only have data for 3 patients of which they have no NfL or CSF PGRN longitudinal data. This is a nothing burger.
Head to Head Passage and Prevail comparison:
Prevail requires symptomatic FTD and sets CDR+NACC FTLD Sum of Boxes 0.5-15 in their inclusion criteria. Passage historically enrolled patients with similar CDR and NACC scores as Prevail did (in their 2024 paper), but are moving to now “enroll patients who are prodromal or have mild cognitive impairment and to exclude patients who are more severely progressed”. This is similar to the INFRONT-2 trial population requiring good health status and no abnormalities on medical exam. It is the at-risk population (n = 16) that INFRONT-3 enrolled. Intervention earlier in the process is better IF the drug can act successfully on the disease process.
Prevail’s genetic elements are made to enable constant high-level expression of PGRN in CNS tissues. Prevail uses a CMV promoter which is susceptible to shutdown by endogenous cellular machinery. However, the CB7 promoter that Passage uses does not have the same shutdown risk. CB7 tends to have more sustained expression compared to CMV in multiple gene therapy contexts. 4
AAV9 used by prevail nonspecifically transduce neurons and non-neuronal cells whereas AAV1 shows specificity for glial cells when given via intracisterna magna injection.
Notably, 60-90% of AAV administered to CSF distributes systemically to major organs regardless of serotype. AAVs are going to circulate in the body and go to the liver and all other organs. A similar proportion of older adults are AAV9 and AAV1 seropositive (73% of adults over 65 have AAV9 antibodies. About 70% of adults have AAV1 antibodies). But, we know that AAV9 has greater systemic toxicity, in part because it is better at transducing cells. Watch out for liver toxicity, and broad inflammatory reactions.
Passage has the better drug, trial design, and focus. Prevail is split amongst a few programs and Lilly has hundreds. This is the least of Lilly’s worries or interests. Here is the only place in Lilly’s Q3 deck where Prevail’s program is mentioned.
Risks to Passage, at a high level, are a) Lilly beats them to market / pivotal trial readout, b) trial recruitment stalls, c) drug doesn’t work. Lilly and Passage are at very similar stages of the process but its been a year since we have heard anything from Lilly about this. Trial recruitment can always stall, but this is why we follow their updates and see how it is going. If Passage’s drug doesn’t work, Prevail’s won’t either. Passage has a market cap of 37M (as of Jan 4, 2026) and cash till Q1 2027 whereas Prevail was bought out for 880M. Passage is the clear play here with 10x upside pending solid clinical data and execution of their Phase 1/2 program.
Closing comments on UniQure and FDA evidence principles
The issue with UniQure was that they did not prespecify their statistical analysis plan. It was not their use of an external propensity matched control. Propensity matching is not randomization and is not a stand in for it. But, when the disease is severe, and we have no disease modifying agents, it is reasonable to use external controls from well-characterized natural history cohorts (e.g., GENFI2 and ALLFTD are the FTD cohorts). The recently released Real World Evidence Principle from FDA along with prior approvals based on natural history data (e.g., Zolengsma) is a clear signal that such external cohorts can be used for comparison. This is good for patients with rare diseases where running placebo-controlled trials is not always possible, ethical, or feasible. Lastly, from a regulation perspective, Will Chou (CEO of Passage) mentioned that the recent rare disease evidence principle from FDA suggests more openness to single arm studies under specific conditions: rare quickly progressing diseases where a drug that addresses genetic cause is available and no other treatments are available. This fits Passage and FTD-GRN well.
What can we look forward to for Passage and what to watch out for?
They plan to have early discussions on experimental design of pivotal trial with FDA in 1H 2026
Does FDA want a placebo arm? Or will an open label Phase 1/2 and ongoing Phase 3 be sufficient?
Data refresh from Phase 1/2 trial in 1H 2026 and will get guidance from FDA after data refresh
What happens to the NfL signal with more patients at the lower dose? Are we able to return NfL changes to that expected of age-related changes?
To what extent does NfL levels correlate with cognitive score levels (i.e., CDR-SB + NACC) over time? This is relevant to the whole field of FTD therapies.
How quickly are they able to enroll patients?
What happens to the cognitive scores over time?
What are the trends in CSF PGRN levels at the lower dose with more patients?
All product made by Catalent, and no need to manufacture any more supply. Next supply will be for registrational cohort so nothing on the manufacturing front for now.
Cash to 1Q 2027, 30M per year burn. Will need to fundraise in the next year, likely off updated Phase 1/2 data.
The cisterna magna is a CSF filled subarachnoid space at the base of the skull. It is a great site for relatively easy access to CSF close to the brain
More specialists can do intracisterna magna injections. Intrathalamic infusions must be done by a neurosurgeon but intracisterna magna injections can be done by neurosurgery or interventional radiology.




This was an excellent, clinician-grade walkthrough of what “success” should actually mean in FTD-GRN: not just moving a biomarker, but stabilizing the neurodegeneration trajectory (NfL/atrophy) early enough to preserve function, because we’re not regrowing neurons, we’re trying to stop the slide.
I also appreciate how clearly you surface the real tradeoffs in the current approaches: AAV gene therapy is conceptually elegant (fix the root cause), but the body’s immune system + systemic biodistribution are not trivial, and “one-time” only counts as a win if the safety profile is truly clean. Meanwhile the indirect strategies (e.g., manipulating trafficking/uptake) can raise plasma markers yet still miss the core intracellular lysosomal biology.
Your point about timing is the one I keep coming back to: the therapeutic window is likely prodromal/very early, before secondary toxic cascades become self-sustaining.
Curious how you think about the clinical trigger to treat in practice: genotype + subtle change, imaging, rising NfL, or a composite?
Thank you!
Great piece! Do you think biomarkers like NfL will end up being the key trigger for when to treat?